Online Database of Chemicals from Around the World
| West Bengal Chemical Industries Limited | India | Inquire | ||
|---|---|---|---|---|
 |
08240399360 | |||
 |
wbcilpvtltd@gmail.com | |||
| Chemical manufacturer since Earl | ||||
| chemBlink standard supplier since 2021 | ||||
| Classification | Biochemical >> Amino acids and their derivatives >> Glycine derivatives |
|---|---|
| Name | Cupric glycinate hydrate |
| Synonyms | Copper 2-aminoacetate;hydrate |
| Molecular Structure | 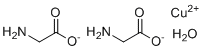 |
| Molecular Formula | C4H10CuN2O5 |
| Molecular Weight | 229.68 |
| CAS Registry Number | 18253-80-0 |
| SMILES | C(C(=O)[O-])N.C(C(=O)[O-])N.O.[Cu+2] |
|
Cupric glycinate hydrate is a coordination compound in which a copper(II) ion is chelated by two glycine ligands, typically with the empirical formula Cu(C2H4NO2)2·xH2O. The glycine ligands coordinate through both the amine nitrogen and the carboxylate oxygen, forming a stable five-membered chelate ring. The hydrate form contains one or more molecules of water of crystallization, which can influence stability, solubility, and appearance. It usually appears as a blue crystalline or powdery solid and is soluble in water. The compound was first prepared in the early 20th century during studies of amino acid–metal interactions, which were of interest both for understanding protein-metal chemistry and for developing nutritional mineral supplements. Researchers found that glycine, the simplest amino acid, forms highly stable complexes with divalent metal ions such as copper(II), making cupric glycinate a convenient form for delivering bioavailable copper. Cupric glycinate hydrate’s primary application is as a dietary copper supplement in human and veterinary nutrition. Copper is an essential trace element involved in hemoglobin synthesis, connective tissue formation, and central nervous system function. In animal feed, it helps prevent growth retardation, anemia, and coat discoloration associated with copper deficiency. Because glycine improves the solubility and intestinal absorption of copper, cupric glycinate is often preferred over inorganic copper salts in certain feed formulations. In biomedical research, cupric glycinate has been investigated for its biochemical roles and possible therapeutic applications. Copper’s redox activity enables it to participate in enzymes like cytochrome c oxidase, superoxide dismutase, and lysyl oxidase. Some studies have examined amino acid–chelated copper complexes for wound healing, antioxidant defense, and anti-inflammatory effects. Cupric glycinate, being more stable and less irritating than copper sulfate, can be advantageous in experimental systems. Industrial uses are more limited but may include use as a trace additive in specialized fermentation media where both copper and nitrogen sources are required, or as a reagent in coordination chemistry studies. Its chelated structure can serve as a model for understanding copper binding in metalloproteins and enzyme active sites. Cupric glycinate hydrate is generally stable under normal conditions but should be stored in a cool, dry place away from strong acids and oxidizing agents. Overheating may cause decomposition with the release of ammonia and nitrogen oxides. While copper is essential, excess intake of cupric glycinate can lead to copper toxicity, manifesting as gastrointestinal upset, liver damage, and, in severe cases, neurological symptoms. Appropriate safety precautions should be followed when handling concentrated forms. References 2018. Metal complexes. US Patent Application, US-2018162803-A1. Priority Date: 2016-12-12 URL: https://patents.google.com/patent/US2018162803A1 2018. Metal complexes. World Patent Application, WO-2018111756-A1. Priority Date: 2016-12-12 URL: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018111756 2009. Hair loss prevention by natural amino acid and peptide complexes. US Patent, US-7834210-B2. Priority Date: 2006-08-04; Grant Date: 2010-11-16 URL: https://patents.google.com/patent/US7834210B2 |
| Market Analysis Reports |
| List of Reports Available for Cupric glycinate hydrate |