Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Organic raw materials >> Heterocyclic compound |
|---|---|
| Name | 2-chloro-7-methyl-7H-Purine |
| Molecular Structure | 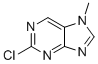 |
| Molecular Formula | C6H5ClN4 |
| Molecular Weight | 168.58 |
| CAS Registry Number | 55286-05-0 |
| EC Number | 867-237-7 |
| SMILES | CN1C=NC2=NC(=NC=C21)Cl |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
2-Chloro-7-methyl-7H-purine is a compound that is used in drug development as a precursor for drug synthesis. 2-Chloro-7-methyl-7H-purine is synthesized by chlorination of 7-methyl-7H-purine under controlled laboratory conditions. The synthesis process involves the reaction of 7-methyl-7H-purine with a chlorinating agent to introduce a chlorine atom at the 2-position of the purine ring. This chemical modification allows one to obtain a chlorinated purine derivative with specific properties suitable for a variety of applications. The molecular structure of 2-chloro-7-methyl-7H-purine has a purine ring with a chlorine atom at the 2-position and a methyl group at the 7-position. This structure gives it specific chemical properties, such as moderate solubility in organic solvents, stability under standard laboratory conditions, and the potential for further functionalization through organic synthesis. In the pharmaceutical field, 2-chloro-7-methyl-7H-purine can serve as a versatile intermediate for the synthesis of biologically active compounds. Its structural features enable modifications to enhance drug potency, improve metabolic stability, and optimize pharmacological properties. Researchers are exploring derivatives of this compound for potential applications in drug discovery programs targeting diseases such as cancer, viral infections, and neurological disorders. From a chemical perspective, 2-chloro-7-methyl-7H-purine can be used as a building block in organic synthesis. Its reactivity allows for the introduction of various functional groups, facilitating the creation of complex molecules and drug intermediates. It is a valuable precursor for the synthesis of fine chemicals, agrochemicals, and specialty materials for various industrial applications. In biological and biomedical research, 2-chloro-7-methyl-7H-purine derivatives are studied for their pharmacological activities and potential therapeutic benefits. The focus is on understanding their mechanisms of action, metabolic pathways, and interactions with biological systems. This research contributes to improved knowledge in drug metabolism, pharmacokinetics, and drug-target interactions, which are essential for the development of effective drug therapies. Future research on 2-chloro-7-methyl-7H-purine aims to explore its applications in personalized medicine, nanotechnology, and targeted drug delivery systems. The focus is on optimizing synthetic methods, expanding its pharmacological properties, and exploring new formulations to address emerging healthcare challenges. |
| Market Analysis Reports |
| List of Reports Available for 2-chloro-7-methyl-7H-Purine |