Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Name | 3,5-Dimethoxy-4-(propan-2-yl)benzoic acid |
|---|---|
| Molecular Structure | 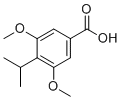 |
| Molecular Formula | C12H16O4 |
| Molecular Weight | 224.25 |
| CAS Registry Number | 55703-81-6 |
| EC Number | 863-594-8 |
| SMILES | CC(C)C1=C(C=C(C=C1OC)C(=O)O)OC |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
|
3,5-Dimethoxy-4-(propan-2-yl)benzoic acid, also known as 4-isopropyl-3,5-dimethoxybenzoic acid, is a well-known organic compound with a wide range of applications in medicinal chemistry and organic synthesis. This benzoic acid derivative has the molecular formula C12H16O4 and is characterized by having methoxy and isopropyl substituents. The synthesis of 3,5-dimethoxy-4-(propan-2-yl)benzoic acid involves the strategic introduction of methoxy (-OCH3) and isopropyl (-CH(CH3)2) groups onto the benzoic acid backbone. The process starts with a precursor such as 3,5-dimethoxybenzaldehyde, attaches the isopropyl group via Friedel-Crafts alkylation or other alkylation techniques, and then oxidizes to convert the aldehyde into a carboxylic acid. 3,5-Dimethoxy-4-(propan-2-yl)benzoic acid is characterized by a benzene ring substituted with methoxy groups at positions 3 and 5 and isopropyl at position 4, along with a carboxylic acid group (-COOH). These substituents give it unique chemical properties, including enhanced lipophilicity and the potential to undergo a variety of chemical reactions. The structure of 3,5-Dimethoxy-4-(propan-2-yl)benzoic acid makes it a valuable intermediate for the synthesis of pharmaceutical compounds. Its substituents can modulate biological activity and enhance the pharmacokinetic properties of drugs. Derivatives of this compound are studied for their potential anti-inflammatory, analgesic, and antimicrobial properties to develop new therapeutic agents. 3,5-Dimethoxy-4-(propan-2-yl)benzoic acid is a versatile building block in organic synthesis, allowing for the creation of more complex molecules through functional group transformations such as esterification, amidation, and reduction. The unique substituents of this compound provide a platform for further modifications, making it useful for the construction of synthetic intermediates for a variety of chemical reactions. In materials science, benzoic acid derivatives, including 3,5-dimethoxy-4-(propan-2-yl)benzoic acid, are used as polymer additives to enhance their properties such as stability, flexibility, and resistance to degradation. The structure of this compound can be studied to understand its potential use in agrochemicals. Its derivatives can be used to develop new pesticides with higher efficacy and environmental characteristics. |
| Market Analysis Reports |
| List of Reports Available for 3,5-Dimethoxy-4-(propan-2-yl)benzoic acid |