Online Database of Chemicals from Around the World
| Shandong Benrite New Chemical Materials Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (0533) 286-6658 | |||
 |
sales@benritechem.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
 |
WeChat: benritechem | |||
 |
WhatsApp: +8613021512891 | |||
| Chemical distributor since 2024 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Organic raw materials >> Aryl compounds >> Biphenyl compounds |
|---|---|
| Name | 2,2'-(1,1'-biphenyl-4,4'-dioxy)diethyl diacrylate |
| Molecular Structure | 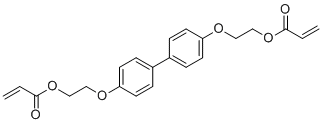 |
| Molecular Formula | C22H22O6 |
| Molecular Weight | 382.41 |
| CAS Registry Number | 90549-12-5 |
| EC Number | 682-828-8 |
| SMILES | C1=CC(=CC=C1C2=CC=C(C=C2)OCCOC(C=C)=O)OCCOC(C=C)=O |
| Hazard Classification | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||
|
2,2'-(1,1'-biphenyl-4,4'-dioxy)diethyl diacrylate is a bifunctional monomer with significant potential in polymer chemistry and material science. This compound is characterized by a biphenyl core, which provides rigidity and thermal stability, linked to two acrylate groups through ethyl spacers. Its chemical structure makes it an attractive candidate for the synthesis of high-performance polymers, coatings, and other advanced materials. The presence of the biphenyl group imparts enhanced mechanical properties, while the acrylate groups enable cross-linking and polymerization. The discovery and development of 2,2'-(1,1'-biphenyl-4,4'-dioxy)diethyl diacrylate emerged from research into multifunctional acrylates that could enhance the properties of polymers used in demanding environments. Acrylate-based compounds have long been studied for their ability to undergo rapid polymerization through free-radical mechanisms. Researchers have sought to improve the strength, flexibility, and thermal resistance of acrylate polymers by introducing rigid aromatic cores like biphenyl. The biphenyl structure in this compound is particularly beneficial because it increases the glass transition temperature and mechanical robustness of the resulting polymers. One of the most notable applications of 2,2'-(1,1'-biphenyl-4,4'-dioxy)diethyl diacrylate is in the field of UV-cured coatings and adhesives. UV-curable resins are widely used due to their fast curing times, low energy requirements, and reduced environmental impact compared to solvent-based systems. The acrylate groups in this compound readily participate in photopolymerization reactions, forming durable and resistant cross-linked networks. The incorporation of the biphenyl core enhances the hardness, scratch resistance, and thermal stability of these coatings, making them suitable for applications in electronics, automotive finishes, and protective layers for optical components. In the realm of high-performance polymers, 2,2'-(1,1'-biphenyl-4,4'-dioxy)diethyl diacrylate is used to produce advanced materials for engineering applications. These polymers exhibit excellent dimensional stability and resistance to heat, chemicals, and mechanical stress. Such properties are critical in industries that require materials capable of withstanding harsh conditions, such as aerospace, electronics, and industrial manufacturing. The rigid biphenyl core also contributes to lower thermal expansion, which is advantageous in applications where maintaining structural integrity under fluctuating temperatures is essential. Another promising application of 2,2'-(1,1'-biphenyl-4,4'-dioxy)diethyl diacrylate is in the development of specialty adhesives. The compound’s ability to polymerize and form strong, cross-linked networks makes it suitable for bonding materials that experience significant mechanical loads or temperature variations. These adhesives can be used in structural bonding, electronic assemblies, and automotive applications, where durability and reliability are paramount. In the field of 3D printing and additive manufacturing, 2,2'-(1,1'-biphenyl-4,4'-dioxy)diethyl diacrylate is gaining attention as a monomer for formulating photopolymer resins. The compound’s fast curing characteristics and ability to produce high-strength polymers make it ideal for creating complex, high-resolution structures. These 3D-printed materials can be used in prototyping, dental applications, and the production of customized components that require precision and robustness. Research into the properties and applications of 2,2'-(1,1'-biphenyl-4,4'-dioxy)diethyl diacrylate continues to expand, driven by the demand for materials with enhanced performance characteristics. The combination of acrylate functionality and the biphenyl backbone positions this compound as a versatile building block in modern polymer science. As industries increasingly seek sustainable and high-performance materials, this compound's role in innovative applications is expected to grow. |
| Market Analysis Reports |
| List of Reports Available for 2,2'-(1,1'-biphenyl-4,4'-dioxy)diethyl diacrylate |