Online Database of Chemicals from Around the World
| Zhohoua Biotech PVT | India | Inquire | ||
|---|---|---|---|---|
 |
+91 9319668540 | |||
 |
zhohouabiotech@zohomail.com | |||
 |
Skype Chat | |||
| Chemical distributor since 2005 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | API >> Nervous system medication >> Other nervous system medication |
|---|---|
| Name | Flualprazolam |
| Synonyms | 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-(1,2,4)triazolo(4,3-a)(1,4)benzodiazepine |
| Molecular Structure | 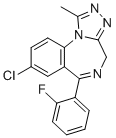 |
| Molecular Formula | C17H12ClFN4 |
| Molecular Weight | 326.76 |
| CAS Registry Number | 28910-91-0 |
| EC Number | 878-264-9 |
| SMILES | CC1=NN=C2N1C3=C(C=C(C=C3)Cl)C(=NC2)C4=CC=CC=C4F |
| Density | 1.4±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 509.8±60.0 ºC 760 mmHg (Calc.)* |
| Flash point | 262.1±32.9 ºC (Calc.)* |
| Index of refraction | 1.698 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H312-H332-H336 Details | ||||||||||||||||||||
| Precautionary Statements | P261-P264-P270-P271-P280-P301+P317-P302+P352-P304+P340-P317-P319-P321-P330-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||
| |||||||||||||||||||||
|
Flualprazolam is a synthetic psychoactive substance belonging to the benzodiazepine class. It is a fluorinated analog of alprazolam, which is widely known as a clinically used anxiolytic and sedative. The structure of flualprazolam includes a triazolo ring fused to a benzodiazepine core, with a fluorine atom substitution that distinguishes it from alprazolam. This modification alters its pharmacological profile, although its general mechanism of action remains consistent with other benzodiazepines, namely acting as a positive allosteric modulator of the γ-aminobutyric acid type A (GABAA) receptor. By enhancing the effect of GABA at these receptors, flualprazolam produces sedative, anxiolytic, muscle relaxant, and anticonvulsant effects. Flualprazolam was first reported in the scientific literature in the late 1970s, when it was synthesized and pharmacologically characterized during the development of benzodiazepine derivatives. Despite its documented activity in experimental studies, it was never introduced to the pharmaceutical market as a therapeutic drug. Instead, it resurfaced decades later as a so-called "designer benzodiazepine," appearing on the unregulated drug market in the mid-2010s. Its availability through online vendors has been associated with non-medical use and public health concerns. The pharmacological activity of flualprazolam has been evaluated in preclinical studies, where it demonstrated high potency in producing sedative and anxiolytic effects. Like other benzodiazepines, it shows dose-dependent central nervous system depression. Reports from toxicological case studies indicate that flualprazolam is active at low oral doses, with effects including drowsiness, impaired coordination, memory disruption, and in higher doses, respiratory depression. Applications of flualprazolam are largely restricted to research and forensic toxicology. It has been identified in biological samples in cases of impaired driving, drug-facilitated crime, and fatal intoxications. Because of its potency, low therapeutic index, and potential for dependence, flualprazolam has raised significant safety concerns. It is not approved for therapeutic use in any country. However, analytical methods such as liquid chromatography–tandem mass spectrometry (LC-MS/MS) have been developed for its detection in biological matrices, enabling forensic laboratories to monitor its occurrence. In response to increasing reports of misuse and associated harms, flualprazolam has been subjected to regulatory controls in multiple jurisdictions. For example, it has been scheduled as a controlled substance in several European countries, North America, and parts of Asia. Its scheduling reflects efforts to curb the spread of designer benzodiazepines that pose risks due to their unknown potency and lack of medical oversight. Flualprazolam illustrates the trajectory of a compound first synthesized in pharmaceutical research but later diverted into the unregulated market. Its rediscovery underscores challenges faced by forensic scientists, toxicologists, and policymakers in monitoring emerging psychoactive substances. While structurally and pharmacologically related to approved benzodiazepines, its unregulated use presents heightened risks, making it a subject of ongoing forensic and public health interest. References 2023. Flualprazolam and flubromazolam: Blood concentrations and prevalence of two novel psychoactive substances in forensic case work in Ontario, Canada. Journal of Analytical Toxicology, 47(7). DOI: 10.1093/jat/bkad058 URL: https://pubmed.ncbi.nlm.nih.gov/37930844 2022. Sensitive detection and primary metabolism analysis of flualprazolam in blood. Journal of Forensic and Legal Medicine, 89. DOI: 10.1016/j.jflm.2022.102388 URL: https://pubmed.ncbi.nlm.nih.gov/35691207 2020. Toxicokinetics and Analytical Toxicology of Flualprazolam: Metabolic Fate, Isozyme Mapping, Human Plasma Concentration and Main Urinary Excretion Products. Journal of Analytical Toxicology, 44(7). DOI: 10.1093/jat/bkaa019 URL: https://pubmed.ncbi.nlm.nih.gov/32104896 |
| Market Analysis Reports |
| List of Reports Available for Flualprazolam |