Online Database of Chemicals from Around the World
| Shanghai Zhuyao Pharmaceutical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (021) 5859-6383 | |||
 |
marketing@pharmalego.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2023 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Organic raw materials >> Heterocyclic compound >> Piperidines |
|---|---|
| Name | 3-(5-Bromo-6-fluoro-1-oxoisoindolin-2-yl)piperidine-2,6-dione |
| Synonyms | 3-(6-bromo-5-fluoro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione |
| Molecular Structure | 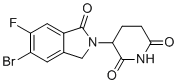 |
| Molecular Formula | C13H10BrFN2O3 |
| Molecular Weight | 341.13 |
| CAS Registry Number | 2409005-96-3 |
| SMILES | C1CC(=O)NC(=O)C1N2CC3=CC(=C(C=C3C2=O)F)Br |
| Density | 1.7±0.1 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.634, Calc.* |
| Boiling Point | 583.6±50.0 ºC (760 mmHg), Calc.* |
| Flash Point | 306.7±30.1 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS07;GHS08 Warning Details
GHS07;GHS08 Warning Details |
|---|---|
| Hazard Statements | H302-H361 Details |
| Precautionary Statements | P201-P202-P264-P270-P281-P301+P312-P308+P313-P330-P405-P501 Details |
| SDS | Available |
|
3-(5-Bromo-6-fluoro-1-oxoisoindolin-2-yl)piperidine-2,6-dione is a chemical compound of significant interest in organic and medicinal chemistry. It belongs to the class of isoindoline derivatives, which have been explored for their diverse biological activities and potential therapeutic applications. The structure of this compound consists of an isoindoline ring system substituted with a bromo-fluoro group at the 5 and 6 positions, respectively, and a piperidine-2,6-dione moiety attached to the nitrogen atom of the isoindoline ring. The functional groups present, including the carbonyl and halogen substitutions, make this compound a valuable intermediate in drug discovery and synthesis. The discovery of 3-(5-Bromo-6-fluoro-1-oxoisoindolin-2-yl)piperidine-2,6-dione can be linked to efforts in exploring the pharmacological potential of isoindoline derivatives, which have been found to exhibit a variety of bioactivities such as anticancer, antimicrobial, and anti-inflammatory properties. The introduction of halogen atoms, such as bromine and fluorine, to the isoindoline core structure is a common strategy to enhance the compound's stability, lipophilicity, and ability to interact with biological targets. The specific arrangement of functional groups in this molecule positions it as a promising candidate for further exploration in the context of drug development. In terms of applications, 3-(5-Bromo-6-fluoro-1-oxoisoindolin-2-yl)piperidine-2,6-dione has shown potential in medicinal chemistry as a scaffold for the development of novel pharmacologically active compounds. The presence of both halogen atoms and the lactam functionality (piperidine-2,6-dione) is beneficial in promoting the compound’s interactions with specific receptors or enzymes involved in various diseases. The fluorine atom is particularly known for its ability to enhance metabolic stability and improve the binding affinity of compounds to their biological targets. The bromo substitution may also contribute to increased hydrophobic interactions, further enhancing the compound's efficacy in biological systems. This compound has been explored in the development of anticancer agents, particularly because of its ability to interact with key enzymes involved in tumor progression. The isoindoline ring system is known to possess a variety of pharmacological activities, including cytotoxicity, which makes it an attractive scaffold for developing selective kinase inhibitors, protease inhibitors, or molecules that interfere with cancer cell signaling pathways. Additionally, the incorporation of the piperidine-2,6-dione ring can lead to enhanced activity and selectivity for certain molecular targets in cancer cells. Moreover, 3-(5-Bromo-6-fluoro-1-oxoisoindolin-2-yl)piperidine-2,6-dione has potential applications in the synthesis of biologically active molecules, such as antimicrobial agents, due to its ability to interfere with microbial growth and cellular processes. The presence of the fluorine atom and the lactam ring might also make this compound useful in the design of inhibitors for specific microbial enzymes, adding value to its therapeutic potential. In addition to its medicinal applications, this compound may also serve as a precursor or intermediate in the synthesis of more complex molecular architectures in material science. The unique structure and functional groups present in 3-(5-Bromo-6-fluoro-1-oxoisoindolin-2-yl)piperidine-2,6-dione may make it suitable for incorporation into functional polymers or for use in organic electronics, where properties such as stability, solubility, and reactivity play a critical role in material performance. Overall, 3-(5-Bromo-6-fluoro-1-oxoisoindolin-2-yl)piperidine-2,6-dione is a promising compound in the field of organic synthesis, drug discovery, and materials science. Its unique combination of functional groups positions it as a valuable intermediate for the development of biologically active molecules with applications in cancer therapy, antimicrobial treatment, and other therapeutic areas. |
| Market Analysis Reports |
| List of Reports Available for 3-(5-Bromo-6-fluoro-1-oxoisoindolin-2-yl)piperidine-2,6-dione |