Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Flavors and spices >> Synthetic spice >> Lactone and oxygen-containing heterocyclic compound >> Thiazole, thiophene and pyridine |
|---|---|
| Name | Ethyl 2-(4-Hydroxyphenyl)-4-methylthiazole-5-carboxylate Hydrochloride |
| Molecular Structure | 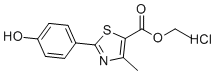 |
| Molecular Formula | C13H14ClNO3S |
| Molecular Weight | 299.77 |
| CAS Registry Number | 399017-10-8 |
| EC Number | 835-656-4 |
| SMILES | CCOC(=O)C1=C(N=C(S1)C2=CC=C(C=C2)O)C.Cl |
| Hazard Symbols |
 GHS05 Danger Details
GHS05 Danger Details | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H318 Details | ||||||||||||
| Precautionary Statements | P264+P265-P280-P305+P354+P338-P317 Details | ||||||||||||
| Hazard Classification | |||||||||||||
| |||||||||||||
| SDS | Available | ||||||||||||
|
Ethyl 2-(4-Hydroxyphenyl)-4-methylthiazole-5-carboxylate hydrochloride is a chemical compound that has gained attention in pharmaceutical and medicinal chemistry due to its unique structural characteristics and potential therapeutic applications. This compound is characterized by the presence of a thiazole ring, a hydroxyphenyl group, and an ethyl ester, each contributing to its biological activity and chemical properties. The discovery of Ethyl 2-(4-Hydroxyphenyl)-4-methylthiazole-5-carboxylate hydrochloride is part of the ongoing exploration of thiazole derivatives, which have long been recognized for their diverse pharmacological properties. Thiazoles are five-membered heterocyclic compounds containing both sulfur and nitrogen atoms, and they are frequently found in bioactive molecules, including antibiotics, anti-inflammatory agents, and antitumor drugs. The introduction of a hydroxyphenyl group at the 2-position of the thiazole ring enhances the compound's potential for interactions with biological targets, particularly enzymes and receptors involved in inflammatory and oxidative stress pathways. Synthetically, Ethyl 2-(4-Hydroxyphenyl)-4-methylthiazole-5-carboxylate hydrochloride can be prepared through a series of reactions that typically involve the formation of the thiazole ring followed by the introduction of the hydroxyphenyl and carboxylate groups. The ethyl ester group not only improves the compound's solubility in organic solvents but also provides a handle for further chemical modifications, such as hydrolysis or esterification, allowing researchers to explore different derivatives with potentially enhanced biological activities. The hydrochloride salt form of the compound improves its solubility in water, making it more suitable for biological assays and potential pharmaceutical formulations. One of the primary applications of Ethyl 2-(4-Hydroxyphenyl)-4-methylthiazole-5-carboxylate hydrochloride is in the development of anti-inflammatory and antioxidant therapies. The compound's structure suggests its ability to inhibit enzymes involved in the production of pro-inflammatory mediators, such as cyclooxygenase (COX) and lipoxygenase (LOX), which are key targets in the treatment of inflammatory diseases. Additionally, the hydroxyphenyl group is known to scavenge free radicals, providing antioxidant activity that could protect cells from oxidative damage, a common feature in chronic diseases like arthritis, cardiovascular disorders, and neurodegenerative conditions. Furthermore, the thiazole ring in Ethyl 2-(4-Hydroxyphenyl)-4-methylthiazole-5-carboxylate hydrochloride may also interact with receptors in the central nervous system, suggesting potential applications in the treatment of neurological disorders. Early studies have indicated that compounds containing thiazole rings can modulate neurotransmitter release or inhibit enzymes that break down neurotransmitters, leading to prolonged effects on mood, cognition, and behavior. These properties make this compound a candidate for further investigation in the context of neuroprotective and psychotropic drug development. The versatility of Ethyl 2-(4-Hydroxyphenyl)-4-methylthiazole-5-carboxylate hydrochloride in medicinal chemistry is further enhanced by its potential use as a lead compound for the synthesis of analogs with improved pharmacokinetic and pharmacodynamic profiles. By modifying the ester or hydroxyphenyl groups, researchers can create a range of derivatives to optimize the compound's stability, bioavailability, and specificity for particular biological targets. This adaptability makes it a promising scaffold for drug discovery and development efforts aimed at addressing a variety of therapeutic needs. In summary, the discovery of Ethyl 2-(4-Hydroxyphenyl)-4-methylthiazole-5-carboxylate hydrochloride highlights the continued importance of thiazole derivatives in pharmaceutical research. Its unique structure, combined with the potential for anti-inflammatory, antioxidant, and neuroprotective applications, ensures that this compound will be a focus of ongoing research in the search for new and effective therapeutic agents. |
| Market Analysis Reports |
| List of Reports Available for Ethyl 2-(4-Hydroxyphenyl)-4-methylthiazole-5-carboxylate Hydrochloride |