Online Database of Chemicals from Around the World
| Natural Micron Pharm Tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (0538) 535-2278 | |||
 |
info@nmpharmtech.com | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Ketones |
|---|---|
| Name | Methylliberine |
| Synonyms | 2-methoxy-1,7,9-trimethylpurine-6,8-dione |
| Molecular Structure | 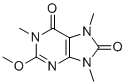 |
| Molecular Formula | C9H12N4O3 |
| Molecular Weight | 224.22 |
| CAS Registry Number | 51168-26-4 |
| SMILES | CN1C2=C(N=C(N(C2=O)C)OC)N(C1=O)C |
| Density | 1.4±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 283.5±50.0 ºC 760 mmHg (Calc.)* |
| Flash point | 125.3±30.1 ºC (Calc.)* |
| Index of refraction | 1.644 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 Details |
| SDS | Available |
|
Methylliberine, also known as Dynamine™, is a purine alkaloid structurally related to caffeine and theacrine. It occurs naturally in several species of coffee, tea, and other plants belonging to the Rubiaceae and Theaceae families. Its chemical structure is defined as 2-methoxy-1,7,9-trimethylpurine-6,8-dione, featuring a substituted xanthine core. The compound attracted scientific and commercial interest due to its potential stimulant effects and its structural similarity to other methylurates. Methylliberine was initially identified as part of phytochemical studies into caffeine-like substances present in Coffea and Camellia species. Its detection was accomplished through chromatographic techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry, often alongside related compounds like theacrine and liberine. While caffeine is typically more abundant in common teas and coffees, methylliberine is present in smaller quantities and was first isolated in its pure form relatively recently during the 21st century, though earlier reports had suggested its presence in exotic plant samples. Commercial interest in methylliberine rose in the dietary supplement and sports nutrition industries due to reports that it offers stimulant-like effects without the habituation commonly associated with caffeine. It is marketed as a cognitive enhancer, often in formulations intended to increase alertness, focus, and energy levels. Anecdotal accounts and preliminary in vitro studies suggested that methylliberine interacts with adenosine receptors and possibly dopamine pathways in a manner similar to caffeine, although these interactions appear to be less potent and may carry a shorter half-life. One of the notable uses of methylliberine is its incorporation into pre-workout blends and nootropic supplements. These products are often combined with caffeine or theacrine to create a synergistic profile aimed at boosting performance or focus. Unlike caffeine, however, methylliberine has been reported to have a more subtle onset and reduced impact on blood pressure and heart rate, which may make it appealing to individuals sensitive to caffeine’s cardiovascular effects. Scientific literature on methylliberine remains limited but growing. Most of the available data focus on its safety profile, pharmacokinetics, and metabolic pathways in humans and animals. Studies have shown that methylliberine is metabolized in the liver, likely involving cytochrome P450 enzymes, especially CYP1A2, which is also responsible for metabolizing caffeine. Importantly, it has been demonstrated that methylliberine does not inhibit this enzyme, suggesting that it does not alter the metabolic clearance of caffeine when co-administered. In pharmacokinetic comparisons, methylliberine has a relatively short half-life, often reported between 1 to 2 hours in healthy adults, in contrast to caffeine’s longer half-life of about 5 hours. This pharmacological property makes methylliberine a suitable option for users seeking acute energy without prolonged stimulant effects or sleep disruption. While methylliberine is not approved as a drug by regulatory agencies such as the FDA, it is generally recognized as a dietary supplement ingredient in jurisdictions where such use is permitted. Toxicological evaluations have indicated a low incidence of adverse effects at standard dosages (50–100 mg), although comprehensive human studies are still lacking. Current regulations vary by country, and in some regions, methylliberine-containing supplements are subject to import or sale restrictions due to the compound's novelty. Despite its growing popularity, the scientific community continues to investigate methylliberine’s long-term effects, its precise mechanism of action, and its comparative efficacy against other xanthine derivatives. Future research may expand its application or define new regulatory boundaries, especially as public interest in natural stimulants and nootropic agents continues to rise. References 2023. Methylliberine Ingestion Improves Various Indices of Affect but Not Cognitive Function in Healthy Men and Women. Nutrients, 15(21). DOI: 10.3390/nu15214509 2022. Effects of caffeine, methylliberine, and theacrine on vigilance, marksmanship, and hemodynamic responses in tactical personnel: a double-blind, randomized, placebo-controlled trial. Journal of the International Society of Sports Nutrition, 19(1). DOI: 10.1080/15502783.2022.2113339 2020. Development of a liquid chromatography-tandem mass spectrometry (LC�MS/MS) method for characterizing caffeine, methylliberine, and theacrine pharmacokinetics in humans. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 1155. DOI: 10.1016/j.jchromb.2020.122278 |
| Market Analysis Reports |
| List of Reports Available for Methylliberine |