Online Database of Chemicals from Around the World
| Hangzhou Zentra Bio-Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
18321999949 | |||
 |
steven@zentrachem.com | |||
 |
QQ chat | |||
 |
WeChat: Hangzhou Zentra Bio-Chemical Co., Ltd. | |||
| Chemical distributor since 2025 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Alcohol |
|---|---|
| Name | 2-Nitro-2-phenyl-1,3-propanediol |
| Molecular Structure | 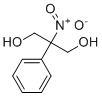 |
| Molecular Formula | C9H11NO4 |
| Molecular Weight | 197.19 |
| CAS Registry Number | 5428-02-4 |
| EC Number | 410-360-4 |
| SMILES | C1=CC=C(C=C1)C(CO)(CO)[N+](=O)[O-] |
| Density | 1.3±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 95 - 96 ºC (Expl.) |
| Boiling point | 396.0±42.0 ºC 760 mmHg (Calc.)* |
| Flash point | 175.7±16.3 ºC (Calc.)* |
| Index of refraction | 1.581 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |


 GHS07;GHS08;GHS09 Danger Details
GHS07;GHS08;GHS09 Danger Details | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H312:-H317:-H372-H411: Details | ||||||||||||||||||||||||||||
| Precautionary Statements | P260-P261-P264-P270-P272-P273-P280-P301+P317-P302+P352-P317-P319-P321-P330-P333+P317-P362+P364-P391-P501 Details | ||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||
|
2-Nitro-2-phenyl-1,3-propanediol is an organic compound with the molecular formula C9H11NO4. It consists of a central carbon atom substituted with a nitro group (–NO2), a phenyl group (–C6H5), and two hydroxymethyl groups (–CH2OH), making it a diol. This structure classifies it as a nitroalcohol and places it within a category of compounds studied for both synthetic utility and pharmacological potential. This compound is often referred to in the literature under the name "nitrodiol" or in some contexts as a derivative of phenylpropanediol. It can be synthesized through the nitration of benzylidene derivatives or by the addition of nitromethane to benzaldehyde followed by further chemical modification. One common method involves the Henry (nitroaldol) reaction between benzaldehyde and nitromethane under basic conditions, which leads to the formation of an intermediate that can be reduced or hydrated to yield 2-nitro-2-phenyl-1,3-propanediol. This compound is of interest primarily due to its stereochemistry and bifunctional reactivity. The central carbon bearing the nitro and phenyl groups is chiral, and the presence of two hydroxyl groups adds to its complexity, offering multiple possibilities for further functionalization. Its ability to form hydrogen bonds through the hydroxyl groups and engage in redox or substitution reactions via the nitro group makes it a valuable intermediate in organic synthesis. In medicinal chemistry, nitroalcohols like 2-nitro-2-phenyl-1,3-propanediol have been studied for their biological activity, including antibacterial and antiparasitic properties. Although this specific compound is not widely used as a therapeutic agent, structurally similar nitro compounds have served as leads for drug discovery. Their biological effects often stem from the nitro group, which can undergo bioreductive activation under physiological conditions, generating reactive species that disrupt cellular functions. Analytically, the compound can be characterized using standard spectroscopic methods. Infrared (IR) spectroscopy reveals strong absorption bands for the nitro group (asymmetric and symmetric N=O stretching) and hydroxyl group O–H stretching. Proton nuclear magnetic resonance (²H NMR) shows signals corresponding to the aromatic protons of the phenyl ring and the diol protons. Carbon-13 NMR (³C NMR) and mass spectrometry are also used to confirm the structure and molecular weight. In terms of applications, 2-nitro-2-phenyl-1,3-propanediol has been used in the synthesis of more complex nitrogen-containing heterocycles and as a building block in organic chemistry research. It may also serve as a model compound in mechanistic studies involving nitro group transformations or chiral alcohol reactivity. The handling of this compound requires standard laboratory safety practices. Nitro compounds may pose risks related to toxicity or sensitization. The presence of hydroxyl groups typically improves the compound’s solubility in polar solvents and may reduce volatility, but it is still advisable to use appropriate protective equipment and work in a well-ventilated area or fume hood. In conclusion, 2-nitro-2-phenyl-1,3-propanediol is a multifunctional organic compound known for its synthetic versatility and structural features. While not broadly applied in pharmaceuticals or industry, it has played a role in synthetic organic chemistry and in the development of nitro-containing bioactive molecules. Its structure offers opportunities for further derivatization and exploration in both academic and applied chemical research. References 2005. The Synthesis and Evaluation of New α-Hydrogen Nitroxides for �Living� Free Radical Polymerization. Synthesis. DOI: 10.1055/s-2005-869894 |
| Market Analysis Reports |
| List of Reports Available for 2-Nitro-2-phenyl-1,3-propanediol |