Online Database of Chemicals from Around the World
| Typu Group | China | Inquire | ||
|---|---|---|---|---|
 |
+86 85220966 | |||
 |
order@typugroup.com | |||
| Chemical distributor since 2008 | ||||
| chemBlink standard supplier since 2021 | ||||
| Classification | Biochemical >> Inhibitor >> Neuronal signaling >> Opioid receptor agonist |
|---|---|
| Name | Dipyanone |
| Synonyms | 4,4-diphenyl-6-pyrrolidin-1-ylheptan-3-one |
| Molecular Structure | 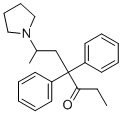 |
| Molecular Formula | C23H29NO |
| Molecular Weight | 335.48 |
| CAS Registry Number | 60996-94-3 |
| SMILES | CCC(=O)C(CC(C)N1CCCC1)(C2=CC=CC=C2)C3=CC=CC=C3 |
| Density | 1.0±0.1 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.557, Calc.* |
| Boiling Point | 479.0±45.0 ºC (760 mmHg), Calc.* |
| Flash Point | 174.7±18.1 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |


 GHS02;GHS06;GHS08 Danger Details
GHS02;GHS06;GHS08 Danger Details |
|---|---|
| Hazard Statements | H225-H301+H311+H331-H370 Details |
| Precautionary Statements | P210-P233-P280-P301+P310-P303+P361+P353-P304+P340+P311 Details |
| SDS | Available |
|
Dipyanone is a synthetic opioid that belongs to the class of 4,4-diphenylheptanone derivatives. Structurally related to methadone, dipyanone shares a similar molecular framework, which includes a central heptanone chain flanked by two phenyl groups. Its molecular formula is C₂₀H₂₅NO, and it was first developed in the mid-20th century during efforts to discover new analgesic drugs. The discovery of dipyanone occurred within the broader context of opioid research, particularly during the 1940s and 1950s when pharmaceutical companies were exploring alternatives to morphine and heroin. Methadone, discovered during World War II, had established the potential of synthetic opioids to manage pain and treat opioid addiction. Building on this work, researchers synthesized dipyanone as a potential analgesic with properties similar to methadone but with modifications to enhance its potency and pharmacokinetics. Dipyanone functions as a mu-opioid receptor agonist, meaning it binds to and activates the same receptors as natural opioids like morphine and endogenous substances like endorphins. This receptor activity results in analgesic effects, making dipyanone effective in pain management. Like other opioids, dipyanone produces its pain-relieving effects by altering the way the brain and nervous system respond to pain signals. Despite its promising analgesic properties, dipyanone never achieved widespread clinical use. It was synthesized and tested during a period when numerous synthetic opioids were being evaluated, and it ultimately fell out of favor in comparison to more established drugs like methadone, which had already demonstrated efficacy in both pain relief and the treatment of opioid dependence. Additionally, the development of new opioids was complicated by growing awareness of the potential for addiction, misuse, and overdose associated with these substances. Dipyanone, like many other synthetic opioids, was eventually restricted to experimental and limited clinical use. In terms of application, dipyanone has been studied primarily for its analgesic effects, with its pharmacological profile suggesting that it could provide long-lasting pain relief due to its extended half-life and sustained activity at the opioid receptors. This made it a candidate for treating chronic pain conditions where sustained opioid therapy might be required. However, its potential for addiction and the emergence of safer alternatives limited its widespread adoption. The challenges surrounding dipyanone’s use mirror those of other opioids, particularly the risks associated with long-term opioid therapy. Tolerance, dependence, and the potential for misuse have been significant concerns, leading to its classification as a controlled substance in many countries. As such, dipyanone is not commonly available in medical practice, and its use is generally confined to research or specialized clinical scenarios where other opioids may not be suitable. Despite its limited application in clinical settings, dipyanone has contributed to the understanding of opioid pharmacology. Its structure and activity have informed the development of other synthetic opioids, and it has been used in research to explore the mechanisms of opioid receptor binding and activation. In particular, studies on dipyanone and similar compounds have helped elucidate the relationship between opioid structure and receptor affinity, which is critical for designing new analgesics with improved safety profiles. In conclusion, while dipyanone did not become a widely used medication, its development highlights the ongoing efforts to discover new opioid analgesics during the 20th century. Its similarities to methadone and its mu-opioid receptor activity demonstrate its potential in pain management, but concerns about addiction and safer alternatives have limited its use. Nonetheless, dipyanone remains of interest in opioid research, particularly in the context of understanding opioid receptor interactions and designing safer analgesics. |
| Market Analysis Reports |
| List of Reports Available for Dipyanone |