Online Database of Chemicals from Around the World
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
 |
Skype Chat | |||
| Chemical distributor | ||||
| chemBlink massive supplier since 2012 | ||||
| International Laboratory Limited | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (650) 278-9963 | |||
 |
admin@intlab.org | |||
| Chemical manufacturer since 2002 | ||||
| Name | Sorivudine |
|---|---|
| Synonyms | 5-[(E)-2-Bromovinyl]-1-[(2R,3S,4S,5R)-3,4-Dihydroxy-5-(Hydroxymethyl)Tetrahydrofuran-2-Yl]Pyrimidine-2,4-Dione; 5-[(E)-2-Bromovinyl]-1-[(2R,3S,4S,5R)-3,4-Dihydroxy-5-(Hydroxymethyl)-2-Tetrahydrofuranyl]Pyrimidine-2,4-Dione; 5-[(E)-2-Bromovinyl]-1-[(2R,3S,4S,5R)-3,4-Dihydroxy-5-Methylol-Tetrahydrofuran-2-Yl]Pyrimidine-2,4-Quinone |
| Molecular Structure | 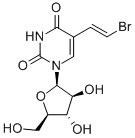 |
| Molecular Formula | C11H13BrN2O6 |
| Molecular Weight | 349.14 |
| CAS Registry Number | 77181-69-2 |
| SMILES | [C@@H]2(N1C=C(C(=O)NC1=O)\C=C\Br)O[C@H](CO)[C@H]([C@@H]2O)O |
| InChI | 1S/C11H13BrN2O6/c12-2-1-5-3-14(11(19)13-9(5)18)10-8(17)7(16)6(4-15)20-10/h1-3,6-8,10,15-17H,4H2,(H,13,18,19)/b2-1+/t6-,7-,8+,10-/m1/s1 |
| InChIKey | GCQYYIHYQMVWLT-HQNLTJAPSA-N |
| (1) | Tapan Sanghvi, Nina Ni, Michael Mayersohn, Samuel H. Yalkowsky. Predicting Passive Intestinal Absorption Using A Single Parameter, QSAR Comb. Sci. 2003, 22(2), 247-257131 compounds and their 'Fraction Absorbed' values have been obtained from the table given in the paper. Some compounds had multiple values reported (as obtained from various references). These have been averaged and provided by us in the SDF and AMP files. The "Sanghvi_values.txt" file contains all data (as reported in Table 1) and "Sanghvi_avg_values.txt" contains averaged data for compounds with multiple entries (as reported in Table 2). "Benserazide, HCl" was retrieved as "Benserazide", "Ceftriaxone, Na" as "Ceftriaxone", "Foscarnet, Na" as "Foscarnet", "L-Dopa" as "Levodopa", "L-Leucine" as "Leucine" and "Triacrilast" as "Tiacrilast". The last compound was found to be mispelt as checked from the original reference. |
|---|---|
| (2) | Matthew D. Wessel, Peter C. Jurs, John W. Tolan, and Steven M. Muskal. Prediction of Human Intestinal Absorption of Drug Compounds from Molecular Structure, J. Chem. Inf. Comput. Sci., 1998, 38(4), 726-735A total of 86 drugs and their %HIA (Human Intestinal Absorption) values taken from the table given in the paper. Compound "trovoflaxicin" was retrieved as "trovafloxacin", "acetylsalicylic acid" as "aspirin", and "phenoxymethylpenicillinic acid" as "penicillinv" from ChemIDplus. Fragment removed from "timolol maleate" during "wash". AMP file has a "Label" column indicating 9 compounds used as a "Cross-Validation Set" and 10 compounds used as an "External Prediction Set" by the authors. Rest of the compounds are labeled as "Training Set". |
| (3) | S. Agatonovic-Kustrin, R. Beresford and A. Pauzi M. Yusof. Theoretically-derived molecular descriptors important in human intestinal absorption, J. Pharm. Biomed. Anal. 2001, 44(12), 1927 - 193786 drugs and their experimentally-derived Intestinal Absorption (%) values have been given. "Fluconasole" was retrieved as "Fluconazole" and "Hydrocortizone" as "hydrocortisone" from ChemIDplus. Drugs were either part of 'Training and testing data sets' or 'Validation data set'. This scheme has been indicated in a 'Label' column in the AMP file. |
| (4) | Zhao YH, Abraham MH, Le J, Hersey A, Luscombe CN, Beck G, Sherborne B, and Cooper I. Rate-limited Steps of Human Oral Absorption and QSAR Studies, Pharm Res., 2002, 19(10), 1446-57 |
| Market Analysis Reports |
| List of Reports Available for Sorivudine |