Online Database of Chemicals from Around the World
| Achemica | Switzerland | Inquire | ||
|---|---|---|---|---|
 |
+41 (24) 466-2929 | |||
 |
contact@achemica.com | |||
| Chemical manufacturer since 2010 | ||||
| Alfa Aesar. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (978) 521-6300 | |||
 |
info@alfa.com | |||
| Chemical manufacturer | ||||
| Alfa Chemistry | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (201) 478-8534 | |||
 |
inquiry@alfa-chemistry.com | |||
| Chemical distributor since 2012 | ||||
| Anward | Hong Kong | Inquire | ||
|---|---|---|---|---|
 |
+852 8191 6999 | |||
 |
sales@anward.com | |||
| Chemical manufacturer since 2012 | ||||
| Beijing LYS Chemicals Co, Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (10) 6841-8738 | |||
 |
jiayanyong@lyschem.com | |||
| Chemical manufacturer since 2004 | ||||
| ChemPacific Corp | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (410) 633-5771 | |||
 |
sales@chempacific.com | |||
| Chemical manufacturer since 1995 | ||||
| International Laboratory Limited | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (650) 278-9963 | |||
 |
admin@intlab.org | |||
| Chemical manufacturer since 2002 | ||||
| King Scientific | USA | Inquire | ||
|---|---|---|---|---|
 |
sales@kingscientific.com | |||
| Chemical manufacturer since 2013 | ||||
| MP Biomedicals LLC. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (440) 337-1200 | |||
 |
info@mpbio.com | |||
| Chemical manufacturer | ||||
| Merck Millipore | USA | Inquire | ||
|---|---|---|---|---|
 |
+86 (10) 5989-8600 | |||
 |
asiatechserv@millipore.com | |||
| Chemical manufacturer since 1954 | ||||
| Name | (Methylsulfanyl)methane |
|---|---|
| Synonyms | (CH3)2S; (Methylsulfanyl)methane #; (methylthio)methane |
| Molecular Structure | 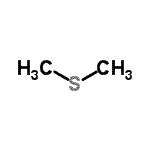 |
| Molecular Formula | C2H6S |
| Molecular Weight | 62.13 |
| CAS Registry Number | 926-09-0 |
| EINECS | 213-133-6 |
| SMILES | CSC |
| InChI | 1S/C2H6S/c1-3-2/h1-2H3 |
| InChIKey | QMMFVYPAHWMCMS-UHFFFAOYSA-N |
| Density | 20 (Expl.) |
|---|---|
| 0.8±0.1g/cm3 (Cal.) | |
| Melting point | -98°C (Expl.) |
| Boiling point | 29.5±3.0°C at 760 mmHg (Cal.) |
| 37.3-37.5°C (Expl.) | |
| Flash point | -36.667°C (Cal.) |
| -36°C (Expl.) | |
| Refractive index | 1.4351 (Expl.) |
| Safety Code | S9;S16;S23;S26;S33;S36/37/39 Details |
|---|---|
| Risk Code | R11;R22;R37/38;R41 Details |
| Hazard Symbol |   X;F Details X;F Details |
| Transport Information | UN1164 |
| Safety Description | DANGER: FLAMMABLE, causes cyanosis, skin and eye irritation |
| Good ventilation, safety glasses. Remove all sources of ignitionfrom the working area. Use in a fume cupboard. DO NOT use in the open lab - thisproduces a long-lived and very unpleasant smell that lin gers! | |
| SDS | Available |
| (1) | Sonia M. Horvat and Carl H. Schiesser. An ab initio and DFT study of homolytic substitution reactions of acyl radicals at sulfur, selenium, and tellurium, New J. Chem., 2010, 34, 1692. |
|---|---|
| Market Analysis Reports |
| List of Reports Available for (Methylsulfanyl)methane |