Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Tianjin Zhongxin Chem-tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (22) 6688-0623 | |||
 |
sales@tjzxchem.com | |||
| Chemical manufacturer since 2007 | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Shanghai Rich Chemicals Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 2025-5798 | |||
 |
william@richchemical.com williamrichchem@hotmail.com | |||
| Chemical manufacturer since 1995 | ||||
| chemBlink standard supplier since 2011 | ||||
| Vesta Intracon Bv | Netherlands | Inquire | ||
|---|---|---|---|---|
 |
+31 (38) 422-2577 | |||
 |
sales@vestachem.com | |||
| Chemical distributor since 1990 | ||||
| chemBlink standard supplier since 2015 | ||||
| Zijun Chemical Industry (Zhongshan) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (760) 8533-8613 | |||
 |
emma@bp-zijun.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2016 | ||||
| Yantai Topway Industrial Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (535) 661-5990 | |||
 |
yttopway@163.com | |||
| Chemical manufacturer since 1998 | ||||
| chemBlink standard supplier since 2016 | ||||
| Classification | Inorganic chemical industry >> Inorganic salt >> Oxides and peroxides >> Non-metal oxides and peroxides |
|---|---|
| Name | Di-tert-butyl peroxide |
| Synonyms | tert-Butyl peroxide; DTBP |
| Molecular Structure | 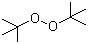 |
| Molecular Formula | C8H18O2 |
| Molecular Weight | 146.23 |
| CAS Registry Number | 110-05-4 |
| EC Number | 203-733-6 |
| SMILES | CC(C)(C)OOC(C)(C)C |
| Density | 0.796 |
|---|---|
| Melting point | -30 ºC |
| Boiling point | 109-110 ºC |
| Refractive index | 1.388-1.390 |
| Flash point | 34 ºF |
| Water solubility | immiscible |
| Hazard Symbols |

 GHS02;GHS08 Danger Details
GHS02;GHS08 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H225-H242-H341 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P203-P210-P233-P234-P235-P240-P241-P242-P243-P260-P264+P265-P271-P273-P280-P284-P303+P361+P353-P304+P340-P305+P351+P338-P318-P337+P317-P342+P316-P370+P378-P403-P403+P235-P405-P410-P411-P420-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 3107 | ||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||
|
Di-tert-butyl peroxide (DTBP) is an organic peroxide used as a free radical initiator and is widely used in polymer chemistry and various industrial processes. Its stability and effectiveness in generating free radicals under heating make it a valuable reagent in numerous chemical reactions. DTBP was synthesized in the early 20th century as part of the exploration of organic peroxides. These compounds gained interest due to their ability to decompose and generate free radicals, which are essential for initiating various chemical reactions. The synthesis of DTBP involves the reaction of tert-butyl hydroperoxide with tert-butyl alcohol. The compound quickly gained recognition due to its stability and effectiveness as an initiator of choice in free radical polymerization processes. The applications of DTBP are very wide, ranging from polymer chemistry to the fuel and pharmaceutical industries. DTBP is widely used as a free radical initiator in polymerization reactions. Its ability to decompose into free radicals at moderate temperatures makes it effective in polymerizing monomers such as ethylene, styrene, and vinyl acetate. This process is essential for the production of various polymers including polyethylene and polystyrene. In the rubber and plastics industry, DTBP acts as a crosslinking agent. It helps to form crosslinked structures in polymers, thereby enhancing their mechanical strength and heat resistance. This application is essential for manufacturing durable rubber products and high-performance plastics. DTBP is used as a cetane improver in diesel fuel. By promoting more complete combustion, it can improve fuel efficiency and reduce emissions. Its role in improving ignition quality is particularly beneficial in cold weather, where diesel engines face difficulties starting. In medicine, DTBP is used as an oxidant in the synthesis of various compounds. Its ability to introduce oxygen into chemical structures is valuable in the production of active pharmaceutical ingredients and intermediates. DTBP, like other organic peroxides, must be handled with care. It is stable under normal conditions but can decompose explosively if exposed to heat or contamination. Proper storage, away from heat and incompatible materials, and the use of protective equipment are essential to ensure safety. References 1948. The Reaction of Di-t-butyl Peroxide and Benzaldehyde. Journal of the American Chemical Society. DOI: 10.1021/ja01190a016 2023. Additives to Biodiesel Fuels. Chemistry and Technology of Fuels and Oils. DOI: 10.1007/s10553-023-01611-8 2024. Enantioselective alkyl-alkyl coupling by Ni-catalysed asymmetric cross-hydrodimerization of alkenes. Nature Synthesis. DOI: 10.1038/s44160-024-00609-2 |
| Market Analysis Reports |
| List of Reports Available for Di-tert-butyl peroxide |