Online Database of Chemicals from Around the World
| E-fine Bio Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 15251778053 | |||
 |
William.efine@hotmail.com | |||
| Chemical manufacturer since 2021 | ||||
| chemBlink standard supplier since 2022 | ||||
| Suzhou Jinghe Pharmaceutical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 15897565406 | |||
 |
yangshu5406@163.com | |||
| Chemical manufacturer since 2023 | ||||
| chemBlink standard supplier since 2026 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyridine compound |
|---|---|
| Name | 3-(4-Hexyloxy-1,2,5-thiadiazol-3-YL)-1-methylpyridinium iodide |
| Synonyms | 3-hexoxy-4-(1-methylpyridin-1-ium-3-yl)-1,2,5-thiadiazole;iodide |
| Molecular Structure | 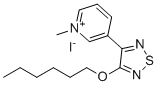 |
| Molecular Formula | C14H20IN3OS |
| Molecular Weight | 405.30 |
| CAS Registry Number | 131988-19-7 |
| SMILES | CCCCCCOC1=NSN=C1C2=C[N+](=CC=C2)C.[I-] |
|
3-(4-Hexyloxy-1,2,5-thiadiazol-3-YL)-1-methylpyridinium iodide is an organic compound that combines a pyridinium cation with a thiadiazole moiety substituted with a hexyloxy group. This compound is characterized by its structure, in which a pyridinium group is attached to a thiadiazole ring, with the thiadiazole group further substituted at the 3-position by a hexyloxy group. The iodide counterion is present to balance the charge of the pyridinium cation. The discovery and synthesis of compounds like 3-(4-hexyloxy-1,2,5-thiadiazol-3-YL)-1-methylpyridinium iodide are of significant interest in organic chemistry, particularly due to their potential in materials science, specifically in the field of conducting organic materials. Pyridinium salts are known to have applications in a variety of fields due to their ability to act as electron-rich species, and the presence of the thiadiazole group in the structure adds further electronic versatility, making the compound a candidate for studies in electronic applications. One of the key applications of this compound is in the development of organic electronic materials. The pyridinium cation, in combination with the thiadiazole group, contributes to the compound's ability to act as a charge carrier or participate in charge transfer processes, which are essential for organic semiconductor development. Thiadiazole derivatives are known for their electronic properties, and the incorporation of the hexyloxy group at the 4-position of the thiadiazole ring can improve solubility and processability, which is beneficial for the fabrication of thin films and other forms of organic electronic devices. Another application of 3-(4-hexyloxy-1,2,5-thiadiazol-3-YL)-1-methylpyridinium iodide is in the field of coordination chemistry. Pyridinium salts can serve as ligands in metal complexation, and the presence of the electron-donating thiadiazole and hexyloxy groups can modulate the coordination properties of the pyridinium cation. Such complexes may have potential applications in catalysis, especially in processes that require electron-rich ligands for metal ions. In addition to their electronic applications, pyridinium-based compounds, particularly those with thiadiazole substitutions, have been studied for their potential biological activities. The incorporation of thiadiazole rings into organic structures has been shown to enhance biological activity in certain contexts, and the present compound might also exhibit some pharmacological or antimicrobial properties. However, as of the latest verified literature, there are limited reports specifically on the biological applications of 3-(4-hexyloxy-1,2,5-thiadiazol-3-YL)-1-methylpyridinium iodide. In summary, 3-(4-hexyloxy-1,2,5-thiadiazol-3-YL)-1-methylpyridinium iodide is an interesting compound that has been synthesized and characterized for its potential applications in organic electronics, coordination chemistry, and possibly pharmacology. Its structure, which combines pyridinium and thiadiazole components, provides a platform for further research and development in various fields of materials science and organic synthesis. References 2019. Xanomeline derivatives and methods for treating neurological disorders. US-2021145810-A1 2019. Xanomeline derivatives and methods for treating neurological disorders. WO-2021097427-A1 2019. Xanomeline derivatives and methods for treating neurological disorders. US-11534434-B2 |
| Market Analysis Reports |
| List of Reports Available for 3-(4-Hexyloxy-1,2,5-thiadiazol-3-YL)-1-methylpyridinium iodide |