Online Database of Chemicals from Around the World
| Hangzhou Verychem Science And Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8816-2785 +86 13606544505 | |||
 |
lucy@verychem.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink massive supplier since 2021 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Tianjin Zhongxin Chem-tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (22) 6688-0623 | |||
 |
sales@tjzxchem.com | |||
| Chemical manufacturer since 2007 | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Ereztech LLC | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (888) 658-1221 | |||
 |
sales@ereztech.com | |||
| Chemical distributor since 2010 | ||||
| chemBlink standard supplier since 2011 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Hangzhou Foreign Economic Relations and Trade Service Co., Ltd | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13957167880 | |||
 |
triggerchem@126.com | |||
 |
QQ chat | |||
| Chemical distributor since 2000 | ||||
| chemBlink standard supplier since 2020 | ||||
| Classification | Inorganic chemical industry >> Inorganic salt >> Metal halides and halides >> Metal chlorides and salts |
|---|---|
| Name | Zirconyl chloride octahydrate |
| Synonyms | Zirconium(IV) oxychloride octahydrate |
| Molecular Structure | 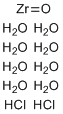 |
| Molecular Formula | ZrOCl2.8(H2O) |
| Molecular Weight | 322.25 |
| CAS Registry Number | 13520-92-8 |
| EC Number | 603-909-6 |
| SMILES | O.O.O.O.O.O.O.O.O=[Zr].Cl.Cl |
| Density | 1.91 |
|---|---|
| Water solubility | SOLUBLE |
| Hazard Symbols |
 GHS05 Danger Details
GHS05 Danger Details | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H314 Details | ||||||||||||||||||||||||||||
| Precautionary Statements | P260-P264-P280-P301+P330+P331-P302+P361+P354-P304+P340-P305+P354+P338-P316-P321-P363-P405-P501 Details | ||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| Transport Information | UN 3260 | ||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||
|
Zirconyl chloride octahydrate (ZrOCl₂·8H₂O) is an inorganic compound of zirconium that has gained prominence in various industrial and chemical applications. This white crystalline solid is a hydrated form of zirconyl chloride, where zirconium is coordinated with chloride ions and water molecules. The compound is notable for its unique properties, making it valuable in several fields, including catalysis, ceramics, and materials science. The discovery of zirconyl chloride can be traced back to the 19th century when researchers began to isolate and study the properties of zirconium compounds. The element zirconium was first isolated in 1824 by the Swedish chemist Jöns Jacob Berzelius. It wasn't until later that compounds like zirconyl chloride were synthesized and characterized. The octahydrate form of zirconyl chloride was identified as a stable and easily handled salt, making it suitable for various applications in both research and industry. Zirconyl chloride octahydrate is primarily used in the production of zirconium oxide (ZrO₂), a material known for its high melting point, strength, and resistance to corrosion. This oxide has significant applications in ceramics, particularly in the manufacturing of porcelain, tiles, and advanced ceramics used in dental applications. Zirconium oxide's properties make it an excellent choice for high-performance materials, including thermal barriers and structural ceramics in aerospace and automotive applications. In addition to its role in ceramics, zirconyl chloride octahydrate serves as a precursor in the synthesis of various zirconium-based catalysts. These catalysts are essential in several chemical processes, including polymerization reactions and the production of specialty chemicals. The catalytic activity of zirconium compounds, including zirconyl chloride, is attributed to their ability to form active sites that facilitate chemical reactions. This property is particularly valuable in the petrochemical industry, where zirconium catalysts are used to produce high-performance polymers and additives. The compound also finds application in analytical chemistry, where it is used as a reagent for the detection of various anions and cations. Zirconyl chloride octahydrate can react with different compounds to form colored complexes, enabling the quantification of specific ions in solution. This property is exploited in various analytical techniques, including spectrophotometry and chromatography. Moreover, zirconyl chloride octahydrate has been investigated for its potential use in biomedical applications. Its biocompatibility and low toxicity make it a candidate for use in drug delivery systems and as a coating for medical devices. Research has shown that zirconium-based materials can enhance the bioactivity of implants, promoting better integration with biological tissues. Environmental applications of zirconyl chloride octahydrate are also emerging. Its ability to adsorb heavy metals and phosphates from wastewater makes it a useful material in remediation processes. The compound can help reduce the environmental impact of industrial effluents by removing toxic substances before they enter water bodies. Despite its versatility and numerous applications, handling zirconyl chloride octahydrate requires caution. The compound can release hydrochloric acid upon hydrolysis, which poses potential hazards. Therefore, proper safety measures should be observed during its storage and use, particularly in laboratory and industrial settings. In summary, zirconyl chloride octahydrate is a valuable inorganic compound with a wide range of applications in ceramics, catalysis, analytical chemistry, and potential biomedical uses. Its discovery has facilitated advancements in materials science and industrial processes, highlighting the importance of zirconium chemistry in modern technology. References 2024. Enhanced degradation of crystal violet dye under solar light by zirconium phosphate-decorated graphitic carbon nitride. Research on Chemical Intermediates, 50(7). DOI: 10.1007/s11164-024-05307-4 2024. Role of synthetic parameters on structure and thermal stability in yttria-stabilized zirconia aerogels. Journal of Sol-Gel Science and Technology, 109(2). DOI: 10.1007/s10971-023-06292-7 2024. Valorization of Furfural to Obtain High Value-Added Products with ZrO2- and Al2O3-Pillared Clays. Topics in Catalysis, 67(13-14). DOI: 10.1007/s11244-024-01971-3 |
| Market Analysis Reports |
| List of Reports Available for Zirconyl chloride octahydrate |