Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Inorganic chemical industry >> Inorganic salt |
|---|---|
| Name | Potassium tetrachloroaurate |
| Molecular Structure | 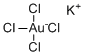 |
| Molecular Formula | KAuCl4 |
| Molecular Weight | 377.87 |
| CAS Registry Number | 13682-61-6 |
| EC Number | 237-190-1 |
| SMILES | Cl[Au-](Cl)(Cl)Cl.[K+] |
| Melting point | 357 ºC (Decomposes) (Expl.) |
|---|---|
| Solubility | Soluble (water) (Expl.) |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||
|
Potassium tetrachloroaurate is an inorganic compound with the chemical formula K\[AuCl4]. It consists of potassium cations (K+) and the tetrachloroaurate anion \[AuCl4]–, where gold is in the +3 oxidation state coordinated to four chloride ions in a square planar geometry. The compound commonly appears as an orange to dark red crystalline solid that is soluble in water and some polar solvents. Potassium tetrachloroaurate is typically prepared by dissolving elemental gold in aqua regia (a mixture of nitric acid and hydrochloric acid), which forms chloroauric acid (HAuCl4). Subsequent neutralization of chloroauric acid with potassium chloride or potassium hydroxide yields potassium tetrachloroaurate as a stable salt. This process is well established and used for the production of gold compounds in both laboratory and industrial settings. Structurally, the gold center in the tetrachloroaurate ion is coordinated by four chloride ligands arranged in a square planar geometry. This coordination environment is characteristic of Au(III) complexes and contributes to the compound’s chemical stability. The potassium ion balances the charge of the anionic complex, and in the solid state, the ions are arranged in a lattice stabilized by ionic interactions. Potassium tetrachloroaurate serves as a key intermediate and reagent in gold chemistry. It is widely used as a precursor for the synthesis of gold catalysts, gold nanoparticles, and other organometallic gold complexes. Due to its solubility and stability, it is convenient for use in aqueous and non-aqueous synthesis. One of the major applications of potassium tetrachloroaurate is in catalysis. Gold(III) complexes derived from this salt catalyze a variety of reactions including oxidation, hydroamination, and coupling reactions in organic synthesis. These gold-based catalysts have gained importance for their selectivity and mild reaction conditions. Potassium tetrachloroaurate is also utilized in the preparation of gold nanoparticles by reduction methods. The controlled reduction of \[AuCl4]– ions allows the formation of gold nanoparticles with specific sizes and shapes, which have applications in electronics, medicine, and materials science. In addition, potassium tetrachloroaurate finds use in analytical chemistry and gold plating processes. Its gold content and solubility make it suitable for gold electrodeposition and as a standard reagent in gold quantification assays. Handling potassium tetrachloroaurate requires standard safety precautions due to its oxidative properties and toxicity. It should be stored in a dry, well-ventilated area, and contact with skin or eyes should be avoided. In summary, potassium tetrachloroaurate is a gold(III) chloride complex salt widely used as a precursor in gold chemistry, catalysis, nanoparticle synthesis, and electroplating. It is typically prepared by neutralizing chloroauric acid with potassium salts. Its chemical stability, solubility, and well-defined structure make it an indispensable reagent in both research and industrial applications involving gold. |
| Market Analysis Reports |
| List of Reports Available for Potassium tetrachloroaurate |