Online Database of Chemicals from Around the World
| Qingdao Free Trade Zone United International Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (532) 83893696 +86 13808950921 | |||
 |
jason.wang@unitedint.com | |||
 |
QQ chat | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2006 | ||||
| Chemtrade International Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (532) 8689-3005 | |||
 |
chemtrade@trade-chem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Shandong Baolingbao Biotechnology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (534) 212-6099 891-8699 212-6077 | |||
 |
baolingbao@blb-cn.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Nile Chemicals | India | Inquire | ||
|---|---|---|---|---|
 |
+91 (22) 6631-3162 | |||
 |
sales@nilechemicals.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Singsino Group Limited | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (532) 8580-8802 | |||
 |
chinaglucose@gmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Asian Winner Group Universal Limited | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (532) 8667-9186 | |||
 |
sales@asianwinnercn.com | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2010 | ||||
| Classification | Biochemical >> Carbohydrate >> Monosaccharide |
|---|---|
| Name | D-(+)-Glucose monohydrate |
| Synonyms | D-(+)-Glucopyranose monohydrate; Glucose; Corn sugar; a-D-Glucose monohydrate; (2S,3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol hydrate |
| Molecular Structure | 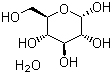 |
| Molecular Formula | C6H12O6.H2O |
| Molecular Weight | 198.17 |
| CAS Registry Number | 14431-43-7 (5996-10-1) |
| EC Number | 604-408-5 |
| SMILES | C(O)[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)O1.O |
| Solubility | 1000 g/L (water 20 ºC |
|---|---|
| Melting point | 83 ºC |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P305+P351+P338 Details |
| SDS | Available |
|
α-D-Glucose monohydrate, a naturally occurring monosaccharide, is a fundamental carbohydrate in biochemistry and a cornerstone of energy metabolism. This compound exists as a cyclic form of D-glucose, a critical sugar in the metabolic pathways of most organisms. The monohydrate form crystallizes with a single water molecule, which stabilizes its structure and is commonly used in research and industrial applications. Its discovery is linked to the broader understanding of carbohydrates developed during the 19th century, particularly the structural elucidation of glucose by Emil Fischer, who received the Nobel Prize in Chemistry in 1902 for his work on sugars. This carbohydrate plays a central role in energy storage and transfer within biological systems. In its natural state, α-D-glucose forms glycosidic linkages in polysaccharides like starch and glycogen, which are hydrolyzed to release glucose as a metabolic substrate. It serves as the primary energy source in glycolysis, a fundamental metabolic pathway. Furthermore, its oxidation in the presence of oxygen generates ATP, providing energy for cellular functions. α-D-Glucose monohydrate is extensively used in laboratory and industrial contexts. In biochemistry and molecular biology, it is a key reagent for studying enzymatic activity, particularly in reactions involving hexokinase, glucose oxidase, and other glucose-metabolizing enzymes. Its crystalline monohydrate form is preferred due to its stability and ease of handling compared to its anhydrous counterpart. In the pharmaceutical industry, α-D-glucose monohydrate finds application as an excipient in tablet formulations, syrups, and injectable solutions. Its role as a stabilizer and bulking agent enhances the efficacy and stability of medicinal products. Additionally, it is used in cell culture media to provide a consistent and bioavailable energy source for growing cells in vitro. The food and beverage industries utilize α-D-glucose monohydrate in various products. It is employed as a sweetener and energy supplement in sports drinks and nutritional formulations. Its controlled hydrolysis produces glucose syrups, which serve as sweeteners and texturizing agents in candies, baked goods, and beverages. Moreover, it acts as a substrate in fermentation processes to produce bioethanol, organic acids, and other value-added products. α-D-Glucose monohydrate also plays a role in clinical diagnostics. It is a standard reference in blood glucose assays and other tests measuring glucose levels in biological samples. These tests are essential for diagnosing and managing conditions like diabetes mellitus, where glucose regulation is impaired. The compound’s importance in both fundamental research and applied science underscores its versatility and utility. Its widespread availability and adaptability have made α-D-glucose monohydrate a staple in multiple domains, from understanding metabolic pathways to supporting industrial processes. References 2024. Modified release of D-glucose incorporated into laponite/ureasil-poly(ethylene oxide) hybrid nanocomposite. Journal of Sol-Gel Science and Technology, 110(2). DOI: 10.1007/s10971-024-06387-9 2020. THz Spectroscopy of Bound Water in Glucose: Direct Measurements from Crystalline to Dissolved State. Journal of Infrared, Millimeter, and Terahertz Waves, 41(4). DOI: 10.1007/s10762-020-00684-4 2020. Determining Honey Adulteration by Seeding Method: an Initial Study with Sunflower Honey. Food Analytical Methods, 13(5). DOI: 10.1007/s12161-020-01711-9 |
| Market Analysis Reports |
| List of Reports Available for D-(+)-Glucose monohydrate |