Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Inorganic chemical industry >> Inorganic salt >> Inorganic ammonium salt |
|---|---|
| Name | Ammonium chloroplatinate |
| Synonyms | Diammonium hexachloroplatinate; Ammonium hexachloroplatinate(IV); Platinum(IV)-ammonium chloride |
| Molecular Structure | 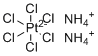 |
| Molecular Formula | (NH4)2.PtCl6 |
| Molecular Weight | 443.88 |
| CAS Registry Number | 16919-58-7 |
| EC Number | 240-973-0 |
| SMILES | [NH4+].[NH4+].Cl[Pt-2](Cl)(Cl)(Cl)(Cl)Cl |
| Density | 3.06 g/mL (Expl.) |
|---|---|
| Hazard Symbols |


 GHS05;GHS06;GHS08 Danger Details
GHS05;GHS06;GHS08 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301-H317:-H318:-H334: Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P233-P260-P261-P264-P264+P265-P270-P271-P272-P280-P284-P301+P316-P302+P352-P304+P340-P305+P354+P338-P317-P321-P330-P333+P317-P342+P316-P362+P364-P403-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 3288 | ||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||
|
Ammonium chloroplatinate, chemically known as (NH4)2PtCl6, is an inorganic coordination compound comprising platinum in the +4 oxidation state complexed by six chloride ligands, balanced by two ammonium cations to maintain electrical neutrality. It typically appears as an orange to yellow crystalline solid that is sparingly soluble in water and insoluble in most organic solvents. This compound has historically played a central role in the processing and refining of platinum metals and continues to be important in both industrial and research applications. The synthesis of ammonium chloroplatinate is most commonly achieved during the recovery and purification of platinum from ore or recycled materials. Platinum-containing solutions, often obtained after dissolving platinum group metals in aqua regia (a mixture of nitric acid and hydrochloric acid), are treated with ammonium chloride. This treatment precipitates ammonium chloroplatinate as a finely divided solid. The precipitation reaction is highly selective for platinum(IV) ions, allowing for effective separation of platinum from other metals and impurities present in the solution. After filtration, the ammonium chloroplatinate can be washed and dried to obtain a pure product. Structurally, ammonium chloroplatinate consists of the hexachloroplatinate(IV) anion, \[PtCl6]2−, where a platinum ion is octahedrally coordinated by six chloride ions. The two ammonium ions balance the negative charge of the complex anion and are held together by ionic interactions and hydrogen bonding in the solid state, resulting in a crystalline lattice. This arrangement lends the compound high thermal stability, with decomposition occurring at elevated temperatures to yield metallic platinum. One of the primary applications of ammonium chloroplatinate is in the preparation of pure platinum metal. The compound can be thermally decomposed under controlled conditions to produce finely divided platinum powder, which is a valuable material in catalysis and electronics. This platinum powder serves as a precursor for fabricating electrodes, catalytic converters, and various platinum-based catalysts used in chemical synthesis, fuel cells, and environmental applications. In addition to its role in platinum refining and catalyst preparation, ammonium chloroplatinate is utilized in materials science research for studying the coordination chemistry of platinum and its compounds. Its well-defined structure makes it a useful reference compound in analytical chemistry and crystallography. Moreover, it has been employed in specialized chemical syntheses, including the preparation of platinum complexes with tailored ligands for homogeneous catalysis and medicinal chemistry. Handling ammonium chloroplatinate requires adherence to appropriate safety protocols. While the compound itself exhibits low volatility, it can pose risks due to its heavy metal content and potential toxicity if ingested or inhaled in powder form. Proper protective equipment such as gloves, safety glasses, and lab coats should be worn, and exposure should be minimized. The compound should be stored in a dry, cool environment, away from strong reducing agents and incompatible chemicals. The compound’s industrial importance is further underscored by its use in quality control during platinum recovery processes. Its formation indicates the presence of platinum in solution, and the efficiency of its precipitation affects the yield and purity of recovered metal. Advances in platinum refining have sought to optimize ammonium chloroplatinate precipitation to improve economic and environmental outcomes. In summary, ammonium chloroplatinate is an orange-yellow crystalline salt consisting of the hexachloroplatinate(IV) anion balanced by ammonium cations. It is chiefly important in platinum extraction, refining, and catalyst precursor preparation. Its distinctive chemical and structural properties underpin its widespread industrial and research applications, making it a vital compound in platinum chemistry. References 2018. Cross-reactivity between halogenated platinum salts in an immediate-type respiratory hypersensitivity model. Inhalation Toxicology, 30(11). DOI: 10.1080/08958378.2018.1554015 2015. Lung function changes in mice sensitized to ammonium hexachloroplatinate. Inhalation Toxicology, 27(11). DOI: 10.3109/08958378.2015.1070219 1999. Interaction of HLA Phenotype and Exposure Intensity in Sensitization to Complex Platinum Salts. American Journal of Respiratory and Critical Care Medicine, 160(2). DOI: 10.1164/ajrccm.160.2.9807065 |
| Market Analysis Reports |
| List of Reports Available for Ammonium chloroplatinate |