Online Database of Chemicals from Around the World
| Topscience Co. Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (400) 820-0310 | |||
 |
sales@tsbiochem.com | |||
| Chemical manufacturer since 2013 | ||||
| chemBlink standard supplier since 2012 | ||||
| Classification | Biochemical >> Inhibitor >> DNA damage |
|---|---|
| Name | AOH1996 |
| Synonyms | N-(2-((2-(3-Methoxyphenoxy)phenyl)amino)-2-oxoethyl)-1-naphthamide |
| Molecular Structure | 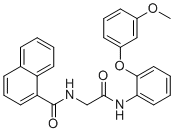 |
| Molecular Formula | C26H22N2O4 |
| Molecular Weight | 426.46 |
| CAS Registry Number | 2089314-64-5 |
| SMILES | COC1=CC(=CC=C1)OC2=CC=CC=C2NC(=O)CNC(=O)C3=CC=CC4=CC=CC=C43 |
| Density | 1.3±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 703.9±50.0 ºC 760 mmHg (Calc.)* |
| Flash point | 379.5±30.1 ºC (Calc.)* |
| Index of refraction | 1.664 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319 Details |
| Precautionary Statements | P501-P270-P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313-P301+P312+P330 Details |
| SDS | Available |
|
AOH1996 is a small-molecule inhibitor developed to selectively target proliferating cell nuclear antigen (PCNA), a critical protein involved in DNA replication and repair. PCNA functions as a sliding clamp that encircles DNA and recruits various proteins essential for DNA synthesis and cell cycle progression. It is ubiquitously expressed in proliferating cells and often overexpressed in a wide range of human cancers, making it a key target for anticancer therapy. AOH1996 was designed based on the understanding that disrupting PCNA-protein interactions could interfere with tumor cell proliferation while sparing normal cells that do not rely on elevated PCNA activity for survival. The discovery of AOH1996 originated from a rational drug development program aimed at identifying selective PCNA inhibitors with minimal toxicity. The compound was engineered to bind to a cancer-associated isoform of PCNA, distinct from the form present in healthy cells. This isoform-specific targeting was made possible by identifying a unique binding pocket exposed in the cancer-associated conformation of PCNA. AOH1996 binds to this site and disrupts PCNA's interaction with multiple replication and repair proteins, such as DNA polymerases and replication factor C, ultimately leading to DNA replication stress and apoptotic cell death in tumor cells. Preclinical studies have demonstrated that AOH1996 exhibits potent anticancer activity across a broad range of solid tumors, including breast, lung, prostate, and brain cancers. Importantly, the compound was shown to induce cell cycle arrest and apoptosis selectively in cancer cells while having limited cytotoxicity in non-dividing or normal cells. This selective activity is attributed to the compound’s design, which favors binding to the cancer-specific form of PCNA that is not present or is less prevalent in healthy tissues. One of the notable aspects of AOH1996’s development is its performance in animal models. In xenograft studies, AOH1996 significantly suppressed tumor growth without causing significant weight loss or observable organ toxicity in mice. These findings support its potential as a chemotherapeutic agent with a favorable therapeutic window. Additionally, AOH1996 showed additive or synergistic effects when combined with other DNA-damaging agents, such as platinum-based drugs or topoisomerase inhibitors, suggesting its utility in combination therapies aimed at overcoming resistance or enhancing efficacy. Mechanistically, AOH1996 acts by impairing the DNA damage tolerance pathways that cancer cells often exploit to survive genotoxic stress. By disrupting PCNA-mediated repair processes, AOH1996 increases the accumulation of DNA damage, leading to replication fork collapse and activation of apoptosis signaling cascades. These effects are particularly pronounced in cancer cells that have defective checkpoint control or rely heavily on PCNA-driven replication for proliferation. Pharmacological profiling of AOH1996 indicates that it is bioavailable when administered orally and maintains sufficient plasma levels to exert antitumor effects in vivo. Its metabolic stability and low clearance rate in animal models further support its development as a drug candidate. Toxicological evaluations have shown no significant hematologic, hepatic, or renal toxicity at therapeutically relevant doses, although comprehensive human safety data are still pending as the compound advances into clinical development. AOH1996 represents a novel strategy in targeted cancer therapy by exploiting a structural vulnerability in a critical replication protein that is differentially expressed in cancer. Its unique mode of action, coupled with selective toxicity and compatibility with existing therapies, positions it as a promising candidate for the treatment of multiple cancer types. Ongoing research aims to further elucidate its molecular interactions, optimize dosing regimens, and evaluate its clinical potential in early-phase human trials. |
| Market Analysis Reports |
| List of Reports Available for AOH1996 |