Online Database of Chemicals from Around the World
| Shanghai Zhuyao Pharmaceutical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (021) 5859-6383 | |||
 |
marketing@pharmalego.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2023 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Piperazine |
|---|---|
| Name | Thalidomide-Piperazine 5-fluoride |
| Synonyms | 2-(2,6-Dioxopiperidin-3-yl)-5-fluoro-6-(piperazin-1-yl)isoindoline-1,3-dione |
| Molecular Structure | 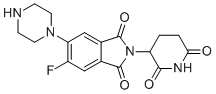 |
| Molecular Formula | C17H17FN4O4 |
| Molecular Weight | 360.34 |
| CAS Registry Number | 2222114-22-7 |
| SMILES | C1CC(=O)NC(=O)C1N2C(=O)C3=CC(=C(C=C3C2=O)F)N4CCNCC4 |
| Density | 1.5±0.1 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.621, Calc.* |
| Boiling Point | 652.2±55.0 ºC (760 mmHg), Calc.* |
| Flash Point | 348.2±31.5 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H315-H319 Details |
| Precautionary Statements | P264-P280-P302+P352-P305+P351+P338-P332+P313-P337+P313-P362 Details |
| SDS | Available |
|
Thalidomide-Piperazine 5-fluoride is a synthetic chemical compound that belongs to the class of compounds known as thalidomide derivatives. Thalidomide itself, a drug originally developed in the 1950s, gained widespread recognition for its sedative properties, but its tragic side effects, particularly teratogenicity (causing birth defects), led to its withdrawal from the market. Despite its controversial past, thalidomide has seen a resurgence in recent years due to its promising applications in the treatment of various medical conditions, including cancer, multiple myeloma, and leprosy. The modification of its structure to create derivatives like Thalidomide-Piperazine 5-fluoride seeks to retain its therapeutic benefits while minimizing its harmful effects. The discovery of Thalidomide-Piperazine 5-fluoride can be traced back to the ongoing research aimed at optimizing thalidomide’s structure for improved efficacy and safety profiles. The inclusion of piperazine, a well-known organic compound often used in medicinal chemistry for its ability to interact with biological systems, serves to enhance the pharmacokinetic properties of the drug. The addition of fluoride in the 5-position is a strategic modification aimed at improving the stability and activity of the compound. Fluorine atoms are frequently incorporated into pharmaceutical compounds to enhance metabolic stability, improve binding affinity to biological targets, and increase the drug’s overall therapeutic potential. In terms of application, Thalidomide-Piperazine 5-fluoride is of particular interest in the treatment of a variety of diseases that require modulation of immune responses. Thalidomide derivatives are known for their ability to suppress inflammatory responses, making them potential candidates for autoimmune diseases such as Crohn’s disease, rheumatoid arthritis, and psoriasis. Thalidomide-Piperazine 5-fluoride, due to its structural modifications, may offer enhanced potency and specificity in targeting inflammatory pathways while avoiding the toxic side effects associated with older thalidomide formulations. Additionally, Thalidomide-Piperazine 5-fluoride could also be explored in the treatment of cancer. Thalidomide and its derivatives have shown promise as anti-cancer agents, particularly in hematologic malignancies such as multiple myeloma. The compound’s ability to inhibit angiogenesis (the formation of new blood vessels that support tumor growth) and its immune-modulatory properties may contribute to its potential efficacy in cancer treatment. The piperazine and fluoride modifications could improve the drug's selectivity and effectiveness in targeting cancer cells, making it a valuable candidate for further clinical investigation. Moreover, the compound could play a role in treating other conditions such as neurological disorders, where immune modulation and anti-inflammatory effects are desired. Thalidomide derivatives have been investigated for their potential use in treating conditions like Alzheimer’s disease and other neurodegenerative disorders, where inflammation and immune dysfunction are key factors in disease progression. The introduction of the piperazine and fluoride groups may enhance the compound’s ability to cross the blood-brain barrier, providing a new avenue for treating such conditions. The development of Thalidomide-Piperazine 5-fluoride is an example of how chemical modifications of existing drugs can lead to new therapeutic possibilities. By refining the structure of thalidomide, researchers aim to overcome the challenges posed by its earlier safety concerns while capitalizing on its therapeutic benefits. This compound’s applications could have significant implications in the fields of immunology, oncology, and neurology, among others. In conclusion, Thalidomide-Piperazine 5-fluoride is a promising chemical derivative of thalidomide that aims to provide safer and more effective treatment options for various medical conditions. Through strategic modifications to the thalidomide molecule, this compound may offer improved pharmacological properties, making it an exciting candidate for further research and development. |
| Market Analysis Reports |
| List of Reports Available for Thalidomide-Piperazine 5-fluoride |