Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Name | 3,3,3-Trifluoro-2-hydroxy-2-(trifluoromethyl)propanenitrile |
|---|---|
| Molecular Structure | 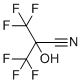 |
| Molecular Formula | C4HF6NO |
| Molecular Weight | 193.05 |
| CAS Registry Number | 677-77-0 |
| SMILES | C(#N)C(C(F)(F)F)(C(F)(F)F)O |
|
3,3,3-Trifluoro-2-hydroxy-2-(trifluoromethyl)propionitrile, commonly referred to as THFP, is a fluorinated organic compound with the chemical formula C4H2F6NO. It has a unique structure that includes a nitrile (-CN) and hydroxyl (-OH) group along with a trifluoromethyl (-CF3) group. The synthesis of THFP stems from efforts to develop compounds with fluorinated motifs because they possess desirable properties such as chemical stability, lipophilicity, and biological activity. The introduction of a trifluoromethyl group into an organic molecule generally enhances these properties, leading to the discovery of THFP. Its synthesis involves the reaction of fluorinated precursors under controlled conditions to achieve the desired structure. The nitrile group provides functional handling for further chemical transformations, while the hydroxyl group increases its reactivity and derivatization potential. THFP is characterized by a propionitrile skeleton substituted with a trifluoromethyl and a hydroxyl group at the 2-position. The presence of the trifluoromethyl group imparts high electronegativity and chemical stability, while the nitrile and hydroxyl groups provide chemical reaction sites. This structure results in a unique combination of properties, such as high stability, resistance to metabolic degradation, and potential for multiple chemical modifications. THFP's unique functionality enables it to participate in a wide range of chemical reactions. The nitrile group can participate in nucleophilic addition reactions, while the hydroxyl group can undergo typical alcohol chemistry reactions, such as esterification or oxidation. The trifluoromethyl group contributes to its overall stability and can affect the reactivity of adjacent functional groups. THFP's stability and lipophilicity make it an attractive building block in drug discovery. It can be incorporated into active pharmaceutical ingredients (APIs) to enhance metabolic stability and alter pharmacokinetic properties, thereby improving the efficacy and safety of the drug. The compound is used in lead compound optimization in medicinal chemistry, where fluorinated motifs are often used to increase the binding affinity and selectivity of drug candidates. THFP is used in the development of agrochemicals such as herbicides and pesticides. Its chemical stability and derivatization potential allow the creation of compounds with enhanced activity and environmental persistence. Derivatives of THFP can serve as effective agents to protect crops from pests and diseases, helping to improve agricultural productivity and sustainability. In materials science, THFP is used to synthesize fluorinated polymers and specialty chemicals. Its incorporation into polymer backbones can impart desirable properties such as enhanced hydrophobicity, thermal stability, and chemical resistance. THFP�s role in the development of advanced materials includes applications in coatings, lubricants, and high-performance composites where fluorinated compounds have superior performance characteristics. THFP is a valuable intermediate in synthetic chemistry, with its functional groups being amenable to a variety of chemical reactions to synthesize complex molecules and fine chemicals through various organic transformations. THFP acts as a reagent in chemical reactions, providing a pathway to introduce trifluoromethyl and nitrile functional groups into target molecules, which are of interest in modern synthetic methods. Due to its fluorinated nature, THFP should be handled with care. Use of appropriate personal protective equipment (PPE) and ventilation is recommended to avoid exposure. |
| Market Analysis Reports |
| List of Reports Available for 3,3,3-Trifluoro-2-hydroxy-2-(trifluoromethyl)propanenitrile |