Online Database of Chemicals from Around the World
| Nanjing Qingze Medical Technology Development Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (570) 5155-7083 5155-7129 | |||
 |
johnnychen2008@gmail.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink standard supplier since 2006 | ||||
| Hunan Geneham Biomedical Technology Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (731) 8555-6298 8514-9956 | |||
 |
geneham@geneham.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Shaanxi Supplier Biological Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (29) 8123-9722 | |||
 |
home@extractsupplier.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Baoji Renshou Chinese Medicine Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (29) 8772-8374 | |||
 |
plant-extract@hotmail.com | |||
| Chemical manufacturer since 2008 | ||||
| chemBlink standard supplier since 2008 | ||||
| Extrasynthese Chemical S.A.S. | France | Inquire | ||
|---|---|---|---|---|
 |
+33 (47) 898-2034 | |||
 |
info@extrasynthese.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Changsha Staherb Natural Ingredients Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (731) 8421-3302 +86 18374838656 | |||
 |
sales06@staherb.cn | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2010 | ||||
| chemBlink standard supplier since 2010 | ||||
| Classification | Biochemical >> Natural biochemical product |
|---|---|
| Name | Ursolic acid |
| Synonyms | 3beta-Hydroxyurs-12-en-28-oic acid |
| Molecular Structure | 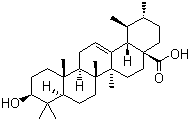 |
| Molecular Formula | C30H48O3 |
| Molecular Weight | 456.71 |
| CAS Registry Number | 77-52-1 |
| EC Number | 201-034-0 |
| SMILES | C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)O)C)C)[C@@H]2[C@H]1C)C)C(=O)O |
| Solubility | insoluble (water), 10 mM (DMSO) |
|---|---|
| Density | 1.1±0.1 g/cm3, Calc.* |
| IMelting point | 292 ºC |
| alpha | 59 º (c=0.3, pyridine) |
| ndex of Refraction | 1.555, Calc.* |
| Boiling Point | 556.9±50.0 ºC (760 mmHg), Calc.* |
| Flash Point | 304.7±26.6 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Safety Description | S24/25 Details | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Classification | |||||||||||||
| |||||||||||||
| SDS | Available | ||||||||||||
|
Ursolic acid is a natural pentacyclic triterpenoid compound found in various plants, particularly in the leaves of apple trees, rosemary, and holy basil. The discovery of ursolic acid can be traced back to the early 19th century when it was first isolated from the leaves of the apple tree (Malus domestica) in 1854 by the chemist E. L. L. Morren. Its name is derived from the Latin word "ursus," meaning bear, because it was originally isolated from the fat of bears. Over time, extensive research has revealed a broad spectrum of biological activities attributed to ursolic acid, making it a subject of interest in pharmacology and nutrition. Ursolic acid is known for its anti-inflammatory, antioxidant, and antimicrobial properties. It acts as a potent inhibitor of the nuclear factor kappa B (NF-kB) signaling pathway, which plays a crucial role in regulating inflammation and immune responses. This activity is significant in the context of various inflammatory diseases, such as arthritis and cardiovascular diseases, where chronic inflammation is a contributing factor. In addition to its anti-inflammatory effects, ursolic acid exhibits notable antioxidant activity. It scavenges free radicals and protects cells from oxidative stress, which is implicated in the aging process and numerous chronic diseases, including cancer and neurodegenerative disorders. Studies have shown that ursolic acid can induce apoptosis (programmed cell death) in cancer cells, making it a potential candidate for cancer therapy. Its effectiveness against various cancer types, including breast, liver, and colon cancers, has been demonstrated in preclinical studies. Ursolic acid also plays a role in metabolic health. Research has indicated that it may enhance muscle growth and strength by promoting the differentiation of muscle cells. This property has garnered attention in the context of sarcopenia, the age-related loss of muscle mass and strength. Furthermore, ursolic acid has been associated with improved insulin sensitivity and lipid metabolism, indicating potential benefits for individuals with metabolic disorders such as obesity and type 2 diabetes. The applications of ursolic acid extend to the cosmetic and food industries as well. Its antioxidant properties make it a valuable ingredient in skincare products, helping to protect the skin from damage caused by UV radiation and pollution. In the food industry, ursolic acid is considered a natural preservative due to its antimicrobial activity, helping to extend the shelf life of various food products. Despite the promising applications of ursolic acid, it is essential to consider the bioavailability of this compound. Studies have shown that ursolic acid has relatively low bioavailability, which may limit its effectiveness when consumed through dietary sources or supplements. However, ongoing research is exploring various formulations and delivery methods to enhance its absorption and therapeutic potential. In conclusion, ursolic acid is a naturally occurring triterpenoid with a rich history of discovery and significant applications in medicine, nutrition, and cosmetics. Its diverse biological activities, particularly its anti-inflammatory and antioxidant properties, position it as a valuable compound in promoting health and preventing disease. References 1979. Phytochemical Investigation of the Flowers of Woodfordia fruticosa. Planta Medica, 36(2). DOI: 10.1055/s-0028-1097262 2024. Role of Terpenoids in Breast Cancer Prevention and Therapy. Current Pharmacology Reports, 10(5). DOI: 10.1007/s40495-024-00377-9 2010. Synthesis and in vitro Cytotoxicity of Novel Ursolic Acid Derivatives. Molecules (Basel, Switzerland), 15(6). DOI: 10.3390/molecules15064033 |
| Market Analysis Reports |
| List of Reports Available for Ursolic acid |