Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Biochemical >> Carbohydrate >> Monosaccharide |
|---|---|
| Name | 1,3,4,6-Tetra-O-acetyl-2-O-trifluoromethanesulfonyl-beta-D-mannopyranose |
| Synonyms | TATM |
| Molecular Structure | 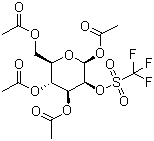 |
| Molecular Formula | C15H19F3O12S |
| Molecular Weight | 480.36 |
| CAS Registry Number | 92051-23-5 |
| EC Number | 688-300-3 |
| SMILES | CC(=O)OC[C@@H]1[C@H]([C@@H]([C@@H]([C@@H](O1)OC(=O)C)OS(=O)(=O)C(F)(F)F)OC(=O)C)OC(=O)C |
| Hazard Symbols |


 GHS05;GHS07;GHS09 Danger Details
GHS05;GHS07;GHS09 Danger Details |
|---|---|
| Hazard Statements | H302-H315-H318-H335-H410 Details |
| Precautionary Statements | P261-P264-P270-P271-P280-P302+P352-P304+P340-P305+P351+P338-P310-P330-P362+P364-P403+P233-P501 Details |
| Transport Information | UN 3077 |
| SDS | Available |
|
1,3,4,6-Tetra-O-acetyl-2-O-trifluoromethanesulfonyl-β-D-mannopyranose is a chemically modified derivative of the sugar mannose, specifically a mannopyranose ring, where four hydroxyl groups at positions 1, 3, 4, and 6 are protected as acetate esters and the hydroxyl group at position 2 is converted into a trifluoromethanesulfonate (triflate) ester. This molecule is a highly functionalized carbohydrate intermediate frequently used in organic synthesis and glycoscience research. The compound was developed to serve as a versatile glycosyl donor precursor. Protecting the hydroxyl groups as acetate esters enhances the compound's stability and solubility in organic solvents, facilitating its handling in synthetic reactions. The triflate group at the 2-position is an excellent leaving group, which makes this derivative particularly reactive for nucleophilic substitution and glycosylation reactions. Synthesis of this tetra-O-acetylated mannopyranose triflate typically begins with D-mannose, which undergoes selective acetylation under controlled acidic conditions to convert the hydroxyl groups into acetate esters. The free hydroxyl at position 2 is then selectively triflated using trifluoromethanesulfonic anhydride (Tf2O) in the presence of a base, usually pyridine, at low temperature to avoid side reactions. The result is a compound where the 2-hydroxyl is replaced by a triflate ester, yielding a reactive electrophile for further synthetic transformations. In carbohydrate chemistry, 1,3,4,6-tetra-O-acetyl-2-O-trifluoromethanesulfonyl-β-D-mannopyranose is widely used as a glycosyl donor for the synthesis of oligosaccharides and glycoconjugates. The triflate group facilitates the formation of glycosidic bonds when treated with nucleophilic acceptors such as alcohols or amines under catalytic or thermal activation. These glycosylation reactions are crucial in assembling complex carbohydrates for biological and medicinal studies, including the development of vaccines, enzyme inhibitors, and glycomimetics. The presence of the acetate protecting groups allows for regioselective deprotection after glycosylation, enabling the stepwise synthesis of oligosaccharides with precise structural control. The stability of the tetra-acetylated sugar during the synthetic steps ensures that the carbohydrate backbone remains intact until the desired transformations are completed. Analytical characterization of this compound includes nuclear magnetic resonance (NMR) spectroscopy, where the acetyl groups exhibit characteristic methyl proton signals around 2 ppm and carbonyl carbon signals near 170–175 ppm in the 1H and 13C NMR spectra, respectively. The triflate group exhibits distinctive signals in 19F NMR spectroscopy, confirming its presence. Infrared (IR) spectroscopy shows strong ester carbonyl stretches near 1750 cm−1 and characteristic triflate S=O stretching bands near 1350–1250 cm−1. Mass spectrometry and elemental analysis are also used to confirm molecular weight and purity. Due to the presence of the highly reactive triflate group, this compound requires careful handling. It is moisture-sensitive and should be stored under inert atmosphere conditions at low temperatures. Exposure to water or nucleophiles can lead to premature cleavage of the triflate and loss of reactivity. In terms of safety, the compound should be handled with appropriate personal protective equipment to avoid skin contact and inhalation of dust or vapors. It is typically used in small quantities in research laboratories specialized in carbohydrate synthesis. Overall, 1,3,4,6-tetra-O-acetyl-2-O-trifluoromethanesulfonyl-β-D-mannopyranose is a well-established synthetic intermediate in glycochemistry. Its design combines protecting groups for stability with a reactive triflate moiety for glycosylation, enabling the efficient assembly of complex carbohydrate structures. This molecule continues to be valuable in research focused on understanding carbohydrate functions and developing carbohydrate-based therapeutics. References 2004. Practical and reliable synthesis of 1,3,4,6-tetra-O-acetyl-2-O-trifluoromethanesulfonyl-beta-D-mannopyranose, a precursor of 2-deoxy-2-[18F]fluoro-D-glucose (FDG). Molecular imaging and biology, 6(5). DOI: 10.1016/j.mibio.2004.06.006 2020. Historical and radiopharmaceutical relevance of [18F]FDG. Journal of Radioanalytical and Nuclear Chemistry, 324(1). DOI: 10.1007/s10967-020-07013-y 2016. 18F-FDG and Other Labeled Glucose Derivatives for Use in Radionuclide Diagnosis of Oncological Diseases (Review). Pharmaceutical Chemistry Journal, 50(5). DOI: 10.1007/s11094-016-1425-y |
| Market Analysis Reports |
| List of Reports Available for 1,3,4,6-Tetra-O-acetyl-2-O-trifluoromethanesulfonyl-beta-D-mannopyranose |