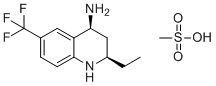
(2R,4S)-2-Ethyl-6-(trifluoromethyl)-1,2,3,4-tetrahydroquinolin-4-amine methanesulfonate is a chemical compound of interest due to its unique molecular structure, which combines a tetrahydroquinoline core with both trifluoromethyl and ethyl groups. These modifications are known to impart specific properties to the molecule, such as enhanced lipophilicity and the potential for selective interactions with biological targets. The stereochemistry of the compound, denoted by (2R,4S), further suggests that the compound may exhibit particular stereospecific biological activity, which could be valuable in drug design.
The synthesis of this compound typically involves the creation of the tetrahydroquinoline ring system, which is achieved through cyclization reactions. The introduction of the trifluoromethyl group at the 6-position and the ethyl group at the 2-position is carried out using selective electrophilic substitution reactions. The methanesulfonate salt form of the compound is produced to enhance its solubility and facilitate its use in biological studies and potential pharmaceutical applications. This synthetic approach ensures that the compound can be obtained with high purity and yield, making it a suitable candidate for further research.
(2R,4S)-2-Ethyl-6-(trifluoromethyl)-1,2,3,4-tetrahydroquinolin-4-amine methanesulfonate has potential applications in medicinal chemistry, particularly as a lead compound for the development of drugs targeting the central nervous system. The trifluoromethyl group is often associated with enhanced metabolic stability, while the ethyl group could influence receptor binding or cellular uptake. These characteristics make the compound a candidate for studies on neuropharmacological activity, such as in the treatment of neurological disorders or as an agent for modulating neurotransmitter systems. The specific stereochemistry could also play a role in its interactions with biological receptors, offering opportunities for the development of highly selective and potent therapeutics.
The compound could be explored in various therapeutic areas, including those related to cognitive function, mood disorders, or even as a modulator of the immune system. Its unique structural features also make it an interesting candidate for further optimization to improve efficacy and reduce side effects.
|