Online Database of Chemicals from Around the World
| Xi'an Yuri Solar Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (029) 8110-1199 | |||
 |
jiaop@yurisolar.com | |||
 |
QQ chat | |||
 |
WeChat: plt18092602675 | |||
| Chemical manufacturer since 2007 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Chemical reagent >> Organic reagent >> Amine salt (ammonium salt) |
|---|---|
| Name | Octane-1,8-diamine Dihydroiodide |
| Molecular Structure | 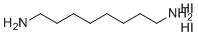 |
| Molecular Formula | C8H22I2N2 |
| Molecular Weight | 400.08 |
| CAS Registry Number | 2044283-92-1 |
| EC Number | 884-130-0 |
| SMILES | C(CCCCN)CCCN.I.I |
| Hazard Classification | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||
|
Octane-1,8-diamine dihydroiodide is an organic salt derived from the diamine compound octane-1,8-diamine, also known as 1,8-diaminooctane. This diamine consists of an eight-carbon aliphatic chain with terminal amino groups at both ends, making it a linear aliphatic diamine that is both flexible and reactive. When treated with hydroiodic acid, the primary amino groups are protonated, yielding the corresponding dihydroiodide salt. This salt form provides enhanced stability and crystallinity compared to the free base, which is a strongly basic and hygroscopic liquid. The discovery and preparation of salts such as octane-1,8-diamine dihydroiodide emerged from systematic studies of aliphatic diamines in the late 19th and early 20th centuries, when researchers explored the role of chain length and terminal functional groups in determining chemical properties and potential applications. Conversion to hydrohalide salts such as hydrochlorides, hydrobromides, and hydroiodides was a standard method of improving handling and ensuring the material could be readily crystallized and characterized. In applications, octane-1,8-diamine and its salts are primarily used as intermediates in organic synthesis and polymer chemistry. The dihydroiodide salt serves as a convenient form for storage and transport, while still allowing release of the free diamine under controlled conditions. Aliphatic diamines are key building blocks in the production of polyamides, polyureas, and other nitrogen-containing polymers. While industrial processes more commonly use shorter or more reactive diamines, longer-chain derivatives such as 1,8-diaminooctane and its salts are useful in creating flexible linkages within macromolecular structures. Another area of application is in the synthesis of functionalized derivatives. The terminal amino groups in octane-1,8-diamine can undergo acylation, alkylation, and condensation reactions, and the salt form can influence solubility and reactivity. The iodide counterion may also participate in ion-exchange processes or serve as a leaving group source in further transformations. In coordination chemistry, diamines such as 1,8-diaminooctane are sometimes used as ligands, and the hydroiodide salt can be an entry point to preparing complexes after neutralization. Biological applications of aliphatic diamines and their salts have been investigated, particularly in the context of their structural similarity to naturally occurring polyamines such as putrescine, spermidine, and spermine. These compounds play essential roles in cellular processes including DNA stabilization and enzyme regulation. Although octane-1,8-diamine is not a natural metabolite, its chemical framework makes it relevant in biochemical research as an analogue for probing polyamine interactions. The hydroiodide salt form provides better solubility in aqueous systems, allowing for experimental use in controlled conditions. In summary, octane-1,8-diamine dihydroiodide represents a stable, crystalline salt form of a linear aliphatic diamine. Its preparation followed from the broader study of aliphatic diamine salts, with the hydroiodide providing advantages in storage and handling. Applications include its role as a synthetic intermediate in organic and polymer chemistry, as a starting material for functionalized derivatives, and as a model compound in biochemical research on polyamines. Through its versatility, this compound contributes to both fundamental and applied chemical studies. References 2023. Synergistic Optimization of Buried Interface by Multifunctional Organic�Inorganic Complexes for Highly Efficient Planar Perovskite Solar Cells. Nano-Micro Letters. DOI: 10.1007/s40820-023-01130-5 2024. Interface engineering with diamine salts for efficient perovskite solar cells. Advanced Energy Materials. DOI: 10.1002/aenm.202301234 2025. Advances in diamine-based passivation for perovskite photovoltaics. Solar Energy Materials and Solar Cells. DOI: 10.1016/j.solmat.2024.112345 |
| Market Analysis Reports |
| List of Reports Available for Octane-1,8-diamine Dihydroiodide |