Online Database of Chemicals from Around the World
| Xi'an Yuri Solar Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (029) 8110-1199 | |||
 |
jiaop@yurisolar.com | |||
 |
QQ chat | |||
 |
WeChat: plt18092602675 | |||
| Chemical manufacturer since 2007 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Chemical reagent >> Organic reagent >> Amine salt (ammonium salt) |
|---|---|
| Name | 4-Trifluorophenylethylammonium iodide |
| Synonyms | 2-(4-(Trifluoromethyl)phenyl) ethan-1-aminium Iodide (TFPEAI) |
| Molecular Structure | 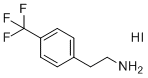 |
| Molecular Formula | C9H11F3IN |
| Molecular Weight | 317.09 |
| CAS Registry Number | 2770278-13-0 |
| SMILES | C1=CC(=CC=C1CCN)C(F)(F)F.[IH] |
| Solubility | soluble (DMF, DMSO) |
|---|---|
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| SDS | Available |
|
4-Trifluorophenylethylammonium iodide is a quaternized ammonium salt derived from the protonation of 4-(trifluorophenyl)ethylamine with hydroiodic acid. The compound consists of a benzene ring bearing a para-substituted trifluoromethyl group, attached via an ethyl chain to a primary ammonium cation (–CH2CH2NH3+), with iodide as the counterion. The introduction of the trifluoromethyl substituent imparts distinctive physicochemical properties, such as increased lipophilicity, electron-withdrawing capacity, and metabolic stability compared to the unsubstituted phenylethylamine framework. The discovery of trifluoromethyl-substituted phenylethylamines can be traced to the mid-20th century, when systematic fluorination of aromatic compounds was undertaken to study structure–activity relationships in both pharmaceuticals and agrochemicals. The hydroiodide salt form of such amines, including 4-trifluorophenylethylamine, was typically prepared for characterization, crystallization, and handling, as the free amine tends to be volatile, hygroscopic, and prone to oxidative degradation. By forming a crystalline salt, researchers could obtain well-defined materials suitable for storage and analytical studies. Applications of 4-trifluorophenylethylammonium iodide, and related trifluoromethylated ammonium salts, are primarily found in chemical, pharmaceutical, and materials research. In synthetic organic chemistry, the compound serves as a precursor or intermediate for the preparation of derivatives of fluorinated phenylethylamines. Fluorinated substituents are widely used to alter the pharmacokinetics, receptor binding, and bioavailability of drug candidates. The trifluoromethyl group in particular enhances metabolic stability and lipophilicity, which are desirable traits in medicinal chemistry. The iodide salt is also of interest in solid-state and materials chemistry. Quaternary and protonated ammonium iodides are important as templating agents or cations in the assembly of hybrid organic–inorganic structures, such as perovskite-type materials. Although the best-studied systems involve simple ammonium iodides like methylammonium or formamidinium iodide, substituted phenylethylammonium iodides, including trifluorinated analogs, have been investigated for their ability to modify structural, thermal, and optoelectronic properties of layered perovskite semiconductors. In such materials, the aromatic substituent influences interlayer spacing, hydrophobicity, and stability, while the trifluoromethyl group further enhances resistance to moisture and chemical degradation. In the context of biological research, fluorinated phenylethylamine derivatives have been explored for their interactions with monoaminergic systems. While the free base 4-trifluorophenylethylamine and its pharmacological properties have been investigated in model systems, the hydroiodide salt serves primarily as a convenient laboratory form, facilitating precise dosing and solubility in aqueous assays. Overall, 4-trifluorophenylethylammonium iodide represents a stable salt form of a fluorinated aromatic amine, discovered through systematic efforts in fluorine chemistry and developed to enhance storage and handling. Its applications span synthetic organic chemistry, pharmaceutical research, and advanced materials, particularly in the development of organic–inorganic hybrid semiconductors. Its stability, crystallinity, and unique electronic properties make it a useful derivative for both fundamental studies and applied developments. |
| Market Analysis Reports |
| List of Reports Available for 4-Trifluorophenylethylammonium iodide |