Online Database of Chemicals from Around the World
| Xi'an Yuri Solar Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (029) 8110-1199 | |||
 |
jiaop@yurisolar.com | |||
 |
QQ chat | |||
 |
WeChat: plt18092602675 | |||
| Chemical manufacturer since 2007 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Chemical reagent >> Organic reagent >> Amine salt (ammonium salt) |
|---|---|
| Name | 3-Fluorophenylethylammonium Iodide |
| Synonyms | 2-(3-fluorophenyl)ethanamine hydroiodide |
| Molecular Structure | 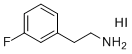 |
| Molecular Formula | C8H11FIN |
| Molecular Weight | 267.08 |
| CAS Registry Number | 2810129-43-0 |
| SMILES | C1=CC(=CC(=C1)F)CCN.I |
|
3-Fluorophenylethylammonium iodide is the hydroiodide salt of 3-fluorophenylethylamine, a compound in which a fluorine atom is substituted at the meta-position of the phenyl ring attached to an ethylamine side chain. In this salt, the amine group is protonated to form the cation –CH2CH2NH3+, balanced by iodide as the counterion. Preparation of the salt typically involves neutralization of 3-fluorophenylethylamine with hydroiodic acid, yielding a crystalline solid that is easier to isolate, purify, and handle compared to the free base. The discovery of fluorinated phenylethylamines, including 3-fluorophenylethylamine, arose during mid-20th century investigations into fluorine-substituted analogs of biologically active aromatic amines. Fluorine substitution was introduced into phenethylamine derivatives to study its effects on receptor affinity, metabolic resistance, and lipophilicity. The iodide salt form was produced mainly for chemical characterization and storage purposes, as ammonium iodides exhibit enhanced stability, lower volatility, and well-defined crystalline forms compared to their free bases. Applications of 3-fluorophenylethylammonium iodide and related salts are primarily found in chemical, pharmaceutical, and materials research. In organic synthesis, such salts serve as intermediates for the preparation of fluorinated analogs of phenylethylamines, which are important scaffolds in medicinal chemistry. The presence of a fluorine atom at the 3-position alters electronic distribution in the phenyl ring and can affect receptor interactions, metabolic stability, and physicochemical properties such as lipophilicity and solubility. In materials chemistry, ammonium iodides including aromatic variants have been investigated as components or templating agents in hybrid organic–inorganic perovskite structures. While most studies focus on simpler salts such as methylammonium iodide and formamidinium iodide, substituted phenylethylammonium iodides, including fluorinated derivatives, have been explored for their ability to modify interlayer spacing, hydrophobicity, and optoelectronic properties in layered perovskite semiconductors. Fluorine substitution enhances chemical stability and may increase the environmental durability of such materials. In biochemical and pharmacological research, fluorinated phenylethylamine derivatives have been examined for their interactions with monoaminergic neurotransmitter systems. Although the iodide salt itself is not directly used in therapy, it provides a stable, well-characterized form of the parent amine suitable for laboratory experiments and precise dosing in aqueous systems. This makes it a useful tool in investigating the structure–activity relationships of phenylethylamine derivatives. Overall, 3-fluorophenylethylammonium iodide is significant as a stable crystalline salt form of a fluorinated phenylethylamine derivative. Its discovery is rooted in the broader context of fluorination chemistry aimed at altering molecular properties, and its applications extend across synthetic chemistry, drug design, and materials science, where fluorine substitution offers distinct advantages in terms of stability and performance. References 2025. Synthesis of zero-dimensional octahedral metal halides through solvent incorporation and their photophysical properties. Nature Chemistry. DOI: 10.1038/s41557-025-01869-x 2024. Fluorinated phenylethylammonium salts for enhanced perovskite solar cell stability. Journal of Materials Chemistry A. DOI: 10.1039/D4TA02345G 2023. Tailoring perovskite interfaces with fluorinated alkylammonium iodides for efficient photovoltaics. Advanced Energy Materials. DOI: 10.1002/aenm.202302345 |
| Market Analysis Reports |
| List of Reports Available for 3-Fluorophenylethylammonium Iodide |