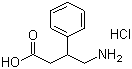
4-Amino-3-phenylbutyric acid hydrochloride, commonly known as Phenibut, is a derivative of the neurotransmitter gamma-aminobutyric acid (GABA). Structurally, it is a beta-phenyl derivative of GABA with a hydrochloride salt form that enhances its solubility and bioavailability. Phenibut was first developed in the Soviet Union in the 1960s, where it was introduced as a pharmaceutical agent with anxiolytic, sedative, and cognitive-enhancing properties. Its unique chemical structure, featuring a phenyl ring, allows it to cross the blood-brain barrier more effectively than GABA itself, providing distinct pharmacological effects.
The discovery of Phenibut arose from efforts to create a compound that could modulate GABAergic signaling while addressing its poor central nervous system penetration. Researchers identified the beta-phenyl modification as a means to increase lipophilicity and facilitate brain uptake. The compound was included in the Soviet cosmonauts' medical kits as an anti-stress agent during space missions, marking its early use in high-pressure scenarios.
4-Amino-3-phenylbutyric acid hydrochloride exerts its effects by acting as a GABA_B receptor agonist and has partial activity at GABA_A receptors. Its ability to modulate GABAergic activity contributes to its anxiolytic, muscle-relaxant, and nootropic effects. Clinically, it has been used in the management of anxiety, insomnia, and vestibular disorders, and as a supplement to enhance cognitive performance. Its calming effects without significant sedative impact have made it a preferred choice in specific therapeutic contexts.
In addition to its clinical applications, Phenibut has gained attention in the dietary supplement industry for its purported benefits in stress reduction and mood enhancement. However, its use outside regulated medical contexts raises concerns about misuse and dependency due to its potential for tolerance and withdrawal symptoms with prolonged use. Regulatory authorities in many countries have restricted its availability, emphasizing the need for careful monitoring of its use.
Ongoing research explores derivatives of Phenibut to optimize its pharmacokinetic and pharmacodynamic profile, aiming to reduce the risk of dependency while retaining therapeutic efficacy. Studies also investigate its potential in treating neurological conditions, including depression, post-traumatic stress disorder, and alcohol withdrawal syndrome.
While 4-Amino-3-phenylbutyric acid hydrochloride remains a compound of significant pharmacological interest, its application highlights the delicate balance between therapeutic benefit and the need for regulation to prevent misuse.
|



















 GHS07 Warning Details
GHS07 Warning Details