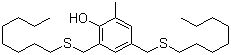
2-Methyl-4,6-bis(octylsulfanylmethyl)phenol is an organic compound that belongs to the class of phenolic antioxidants. With a complex structure characterized by the presence of multiple octylsulfanylmethyl groups, this compound has garnered attention for its unique chemical properties and various applications in industrial processes, particularly in the formulation of stabilizers and additives.
The discovery of 2-methyl-4,6-bis(octylsulfanylmethyl)phenol can be traced back to research focused on the development of effective antioxidants to enhance the stability of polymers and other materials. While specific details about the initial synthesis and characterization of this compound may be limited, the compound is generally synthesized through the reaction of phenolic compounds with alkyl sulfide derivatives, allowing for the incorporation of octyl groups into the molecular structure. This synthetic approach provides a pathway to tailor the properties of the resultant compound for specific applications.
One of the primary applications of 2-methyl-4,6-bis(octylsulfanylmethyl)phenol is as an antioxidant in the polymer industry. The presence of multiple sulfanylmethyl groups imparts enhanced stability and resistance to oxidative degradation in various polymer formulations. This property is crucial for extending the lifespan and performance of materials used in a wide range of applications, including automotive parts, packaging materials, and consumer goods. By inhibiting oxidation, this compound helps maintain the physical and mechanical properties of polymers, ensuring their durability over time.
In addition to its use in the polymer industry, 2-methyl-4,6-bis(octylsulfanylmethyl)phenol is employed in the formulation of lubricants and hydraulic fluids. The compound's antioxidant properties contribute to the thermal stability and performance of these products, preventing degradation and maintaining their efficacy under high-temperature conditions. This application is particularly important in industrial machinery and automotive systems, where lubricant stability is vital for operational efficiency and longevity.
Moreover, this compound is also investigated for potential applications in the cosmetics and personal care industry. Its antioxidant properties may offer benefits in formulations designed to protect skin and hair from oxidative stress caused by environmental factors. By incorporating 2-methyl-4,6-bis(octylsulfanylmethyl)phenol into personal care products, manufacturers can enhance the stability and efficacy of formulations, contributing to the overall performance of cosmetic products.
Despite its beneficial applications, 2-methyl-4,6-bis(octylsulfanylmethyl)phenol is subject to ongoing research to fully understand its safety profile and potential environmental impact. As with many chemical compounds, it is essential to assess the effects of long-term exposure and degradation products to ensure its use aligns with safety and sustainability standards. Proper handling and safety measures are recommended during its production and application in industrial settings to minimize health risks.
In conclusion, 2-methyl-4,6-bis(octylsulfanylmethyl)phenol is an important phenolic antioxidant with diverse applications in the polymer, lubricant, and cosmetics industries. Its discovery and subsequent development have led to its use as a stabilizing agent that enhances the performance and longevity of various materials. As research continues to explore its full potential and address safety considerations, this compound is expected to remain a valuable resource in various industrial applications.
|

















 GHS07 Warning Details
GHS07 Warning Details