Online Database of Chemicals from Around the World
| Fenhe Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (021) 3392-6068 | |||
 |
julius.wei@fenhechem.com | |||
| Chemical manufacturer since 1997 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Amine |
|---|---|
| Name | 4-Amino-3-chlorobenzenethiol |
| Molecular Structure | 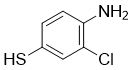 |
| Molecular Formula | C6H6ClNS |
| Molecular Weight | 159.64 |
| CAS Registry Number | 1122-40-3 |
| SMILES | C1=CC(=C(C=C1S)Cl)N |
| Density | 1.4±0.0 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.672, Calc.* |
| Boiling Point | 278.9±0.0 ºC (760 mmHg), Calc.* |
| Flash Point | 122.5±0.0 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
|
4-Amino-3-chlorobenzenethiol is a chemical compound of notable interest in both synthetic chemistry and material science due to its distinctive structure and diverse applications. This molecule features an aromatic benzene ring with an amino group, a chlorinated position, and a thiol group, which contributes to its unique chemical properties. The discovery of 4-Amino-3-chlorobenzenethiol can be traced to efforts in the study of functionalized aromatic compounds. The compound includes a chlorobenzenethiol framework with an amino group at the para position relative to the thiol group. This specific arrangement influences its reactivity and interaction with other chemicals, making it a valuable intermediate in various chemical processes. In synthetic chemistry, 4-Amino-3-chlorobenzenethiol is used as a building block for creating more complex molecules. The presence of both amino and thiol groups in its structure makes it a versatile reagent for various organic reactions, including nucleophilic substitutions and cross-coupling reactions. Its ability to form stable bonds with other functional groups enables the synthesis of compounds with diverse applications. One of the key applications of 4-Amino-3-chlorobenzenethiol is in the development of specialty materials. Its thiol group can react with metal ions to form thiolate complexes, which are useful in materials science for creating new types of catalysts and sensors. These complexes can be used in electronic devices, environmental monitoring, and as catalysts in chemical reactions. Additionally, 4-Amino-3-chlorobenzenethiol has potential applications in medicinal chemistry. The compound’s structure allows it to act as a precursor in the synthesis of pharmaceuticals and biologically active molecules. The amino and thiol groups can be utilized to develop drugs with specific targeting properties or to enhance the activity of existing therapeutic agents. Furthermore, 4-Amino-3-chlorobenzenethiol's unique combination of functional groups makes it an interesting candidate for research into new chemical reactions and materials. Its reactivity can be harnessed to explore new methodologies in organic synthesis or to develop novel materials with tailored properties. In summary, 4-Amino-3-chlorobenzenethiol is a versatile compound with significant implications in synthetic chemistry, materials science, and medicinal research. Its functional groups offer numerous opportunities for creating complex molecules and developing new materials, underscoring its importance in advancing both industrial and academic research. |
| Market Analysis Reports |
| List of Reports Available for 4-Amino-3-chlorobenzenethiol |