Online Database of Chemicals from Around the World
| Hangzhou Verychem Science And Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8816-2785 +86 13606544505 | |||
 |
lucy@verychem.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink massive supplier since 2021 | ||||
| Shanghai Conson Industrial Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6354-8358 | |||
 |
sales@consonchem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Shandong Haoyuan Group | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (536) 519-9628 | |||
 |
fenginfo@gmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2008 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Ring Specialty Chemicals Inc. | Canada | Inquire | ||
|---|---|---|---|---|
 |
+1 (416) 493-6870 | |||
 |
info@ringchemicals.com | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2010 | ||||
| Jiuruibiology & Chemistry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 18867282999 | |||
 |
sales16@jiuruibiochem.com | |||
| Chemical manufacturer since 1996 | ||||
| chemBlink standard supplier since 2015 | ||||
| Chengdu Biopurify Phytochemicals Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (28) 8263-3860 8263-3987 | |||
 |
sales@biopurify.com biopurify@gmail.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2017 | ||||
| Classification | Chemical reagent >> Organic reagent >> Aromatic acid |
|---|---|
| Name | 3,4,5-Trimethoxybenzoic acid |
| Synonyms | Gallic acid trimethyl ether |
| Molecular Structure | 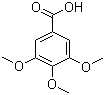 |
| Molecular Formula | C10H12O5 |
| Molecular Weight | 212.20 |
| CAS Registry Number | 118-41-2 |
| EC Number | 204-248-2 |
| SMILES | COC1=CC(=CC(=C1OC)OC)C(=O)O |
| Density | 1.2±0.1 g/cm3, Calc.* |
|---|---|
| Melting point | 168-171 ºC (Expl.) |
| Index of Refraction | 1.524, Calc.* |
| Boiling Point | 376.3±0.0 ºC (760 mmHg), Calc.*, 225-227 ºC (10 mmHg) (Expl.) |
| Flash Point | 128.8±20.0 ºC, Calc.* |
| Water solubility | SLIGHTLY SOLUBLE |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||
|
3,4,5-Trimethoxybenzoic acid is an organic compound that belongs to the class of benzoic acid derivatives, characterized by the presence of three methoxy (-OCH3) groups attached to the benzene ring. It appears as a white crystalline solid with moderate solubility in organic solvents and limited solubility in water. This compound has gained attention due to its applications in pharmaceuticals, agrochemicals, and material science, as well as its role as a key intermediate in organic synthesis. The discovery of 3,4,5-trimethoxybenzoic acid is linked to research on natural and synthetic aromatic carboxylic acids. It can be synthesized through various methods, including the oxidation of 3,4,5-trimethoxytoluene or the carboxylation of 3,4,5-trimethoxyphenyl derivatives. These synthetic routes have been refined to enhance efficiency and yield, allowing large-scale production for industrial applications. One of the primary uses of 3,4,5-trimethoxybenzoic acid is in pharmaceutical research and drug development. It serves as a crucial intermediate in the synthesis of bioactive molecules, particularly those with anti-inflammatory, antimicrobial, and anticancer properties. Several drug candidates and therapeutic agents incorporate the trimethoxybenzoic acid moiety to improve pharmacokinetic properties and biological activity. The compound also plays a significant role in agrochemical formulations. It is used as a precursor for plant growth regulators and herbicides, contributing to enhanced agricultural productivity. Its structural similarity to certain naturally occurring plant metabolites allows it to be utilized in biochemical studies related to plant physiology and metabolism. In material science, 3,4,5-trimethoxybenzoic acid is explored for its potential in polymer chemistry and surface modification. It is employed in the preparation of functionalized materials with specific electronic and optical properties. Its derivatives are used to modify the surface characteristics of nanoparticles, coatings, and bioactive films, providing improved stability and functionality. Analytical chemistry applications of 3,4,5-trimethoxybenzoic acid include its use as a reference compound in chromatography and spectroscopy. Its well-defined molecular structure and stability make it suitable for method development and calibration in analytical techniques such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy. Safety considerations for handling 3,4,5-trimethoxybenzoic acid are similar to those of other benzoic acid derivatives. While it is generally regarded as a low-hazard compound, direct contact may cause mild irritation to the skin and eyes. Proper handling practices, including the use of personal protective equipment, are recommended in laboratory and industrial settings. Future research on 3,4,5-trimethoxybenzoic acid aims to expand its applications in drug discovery, material science, and sustainable chemistry. Advances in green synthetic methods and biocatalysis are being explored to enhance its production efficiency and environmental compatibility. The continued study of its derivatives and functionalized analogs is expected to lead to new applications in various scientific and industrial fields. References 2024. Synthesis of Amide Derivatives of Pyrimidine-Triazolo[1,5-a]pyridin-7-yl) Thiazoles: In Vitro Anticancer Evaluation and In Silico Molecular Binding Studies. Arabian Journal for Science and Engineering. DOI: 10.1007/s13369-024-09685-0 2023. Multimodal action of KRP203 on phosphoinositide kinases in vitro and in cells. Biochemical and Biophysical Research Communications. DOI: 10.1016/j.bbrc.2023.08.050 1960. Potential Reserpine Analogues: Part II. 3,4,5-Trimethoxybenzoic Acid Derivatives. The Journal of Pharmacy and Pharmacology. DOI: 10.1111/j.2042-7158.1960.tb12634.x |
| Market Analysis Reports |
| List of Reports Available for 3,4,5-Trimethoxybenzoic acid |