Online Database of Chemicals from Around the World
| Qingdao Haisu New Material Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13184133710 | |||
 |
alice-haisu@outlook.com | |||
 |
QQ chat | |||
 |
WeChat: 13184133710 | |||
 |
WhatsApp: 13184133710 | |||
| Chemical manufacturer since 2022 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Organic raw materials >> Organosilicon compound |
|---|---|
| Name | Thiol-Derivatized Silica Gel (50 microm) |
| Synonyms | SiliaMetS Thiol |
| Molecular Structure | 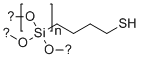 |
| CAS Registry Number | 1189056-65-2 |
| SMILES | [Si](CCCCS)(O[*])(O[*])O[*] |
|
Thiol-derivatized silica gel (50 μm) is a chemically modified form of silica widely used in chromatography, metal ion adsorption, and selective chemical separations. This material is created by functionalizing the surface of porous silica particles with thiol (−SH) groups through covalent bonding. The base silica used typically has a particle diameter of approximately 50 micrometers, which balances column efficiency and backpressure, making it suitable for preparative and analytical applications. The development of thiol-modified silica gels traces back to advancements in surface chemistry during the 1970s and 1980s, when interest in functionalized chromatographic supports expanded. Silica gel, a form of silicon dioxide with a high surface area and mechanical stability, was found to be an ideal substrate for surface modification. By introducing reactive silane coupling agents bearing thiol groups, chemists were able to tailor the chemical properties of the silica surface to interact selectively with target analytes, especially soft metal ions and certain organic compounds. The preparation of thiol-derivatized silica involves the reaction of activated silica gel with organosilanes containing thiol functionalities, such as 3-mercaptopropyltrimethoxysilane. The silanization reaction forms stable Si−O−Si linkages, anchoring the thiol groups to the silica surface. These thiol groups serve as reactive sites capable of forming coordinate bonds with transition metals such as mercury (Hg2+), cadmium (Cd2+), lead (Pb2+), and silver (Ag+), enabling selective extraction or purification. One of the most important applications of thiol-derivatized silica gel is in solid-phase extraction (SPE) for environmental and analytical chemistry. In these methods, the silica gel is packed into cartridges or columns and used to isolate trace amounts of heavy metals from aqueous samples. The high affinity of sulfur atoms in the thiol groups for soft acid metal ions results in efficient binding, even at low concentrations. This makes thiol silica an effective tool in monitoring environmental pollutants and in purifying drinking water or industrial wastewater. In high-performance liquid chromatography (HPLC), thiol-functionalized silica phases are used in both normal-phase and reversed-phase systems for the selective separation of polar or metal-chelating compounds. These columns can also serve as supports for immobilized metal affinity chromatography (IMAC), where the thiol groups are pre-loaded with metal ions to capture proteins, peptides, or other biomolecules that contain histidine, cysteine, or other coordinating residues. Beyond chromatography, thiol silica gels are used in catalysis and material science. The thiol groups can act as anchoring sites for catalytically active metals or organometallic complexes, facilitating their immobilization on a solid support. This approach enables the creation of heterogeneous catalysts with high activity and recyclability, suitable for industrial processes and fine chemical synthesis. Another significant use of thiol silica is in sensor development. Due to their ability to selectively bind metal ions, thiol-functionalized silica particles are used in chemical sensing platforms for detecting toxic metals. These systems often exploit changes in optical or electrochemical properties upon metal binding to generate detectable signals. In biotechnology, thiol silica gels serve as supports for biomolecule immobilization through disulfide bond formation or thiol-maleimide coupling. These techniques are employed in the development of biosensors, affinity purification matrices, and controlled drug delivery systems. The 50 µm particle size represents a practical compromise between flow dynamics and surface area. It is large enough to allow efficient column packing and manageable pressure drops in flow systems, yet small enough to maintain good contact area and surface reactivity. This particle size is commonly used in preparative SPE and mid-pressure chromatography systems. Handling of thiol silica gels requires attention to moisture and oxidation. Thiol groups can oxidize to form disulfides, which reduces their reactivity and binding capacity. Therefore, these materials are typically stored under inert conditions and used promptly after opening. Thiol-derivatized silica gel (50 μm) remains a valuable and versatile tool in modern separation science and material chemistry. Its well-characterized preparation, high selectivity for soft metal ions, and adaptability to various formats ensure its ongoing utility across a range of scientific and industrial applications. |
| Market Analysis Reports |
| List of Reports Available for Thiol-Derivatized Silica Gel (50 microm) |