Online Database of Chemicals from Around the World
| Henan Ouber Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (371) 6532-2607 +86 18937141980 | |||
 |
anna.zhang@oubertec.com | |||
 |
QQ chat | |||
 |
WeChat: 18937141980 | |||
| Chemical manufacturer since 2020 | ||||
| chemBlink massive supplier since 2020 | ||||
| Wudi Reaction Pharma & Chemical Co. Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (0543) 225-7986 +86 15306493269 | |||
 |
sales@ruixinchem.com linacaiwu@163.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2005 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Chemical reagent >> Organic reagent >> Amide |
|---|---|
| Name | N-(5-Methoxy-2-Phenoxyphenyl)methanesulfonamide |
| Molecular Structure | 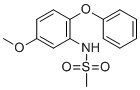 |
| Molecular Formula | C14H15NO4S |
| Molecular Weight | 293.34 |
| CAS Registry Number | 123664-84-6 |
| EC Number | 987-145-1 |
| SMILES | COC1=CC(=C(C=C1)OC2=CC=CC=C2)NS(=O)(=O)C |
| Density | 1.3±0.1 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.599, Calc.* |
| Boiling Point | 411.4±55.0 ºC (760 mmHg), Calc.* |
| Flash Point | 202.6±31.5 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H332-H335 Details |
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P317-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details |
| SDS | Available |
|
N-(5-Methoxy-2-phenoxyphenyl)methanesulfonamide is an organic compound with a sulfonamide functional group and an aromatic moiety. Its unique molecular structure combines methoxy and phenoxy substituents with a sulfonamide group, offering diverse chemical and biological properties. This compound has attracted interest in the fields of medicinal chemistry and materials science due to its potential applications. The discovery of N-(5-methoxy-2-phenoxyphenyl)methanesulfonamide is rooted in the exploration of sulfonamides, which have been pivotal in drug development since their introduction as antimicrobial agents in the 1930s. Over time, the incorporation of diverse substituents onto the aromatic rings of sulfonamides has yielded compounds with enhanced biological activities and expanded their utility into other domains, such as anticancer and anti-inflammatory therapies. This compound's structural framework is particularly relevant for medicinal chemistry due to its capacity to interact with various biological targets. The methanesulfonamide moiety can form hydrogen bonds with active sites of enzymes or receptors, while the aromatic substituents provide hydrophobic interactions and electronic effects. These features make N-(5-methoxy-2-phenoxyphenyl)methanesulfonamide a candidate for therapeutic applications. Preliminary studies suggest that this compound exhibits promising anti-inflammatory activity by modulating key enzymes in the inflammatory cascade, such as cyclooxygenase (COX) or lipoxygenase (LOX). It has been evaluated in vitro for its ability to inhibit prostaglandin synthesis, a hallmark of inflammation. Additionally, the presence of a methoxy group is known to influence bioavailability and metabolism, potentially improving the pharmacokinetic profile of the compound. In the context of oncology, sulfonamide derivatives like N-(5-methoxy-2-phenoxyphenyl)methanesulfonamide have been investigated for their antiproliferative effects. Some studies indicate that these compounds can interfere with cell cycle progression and induce apoptosis in cancer cells by targeting specific signaling pathways. Further research is ongoing to understand their mechanisms of action and optimize their therapeutic potential. This compound may also have utility in the development of functional materials. The sulfonamide group is known for its thermal stability and ability to engage in hydrogen bonding, which could be advantageous in designing polymers or coatings with unique properties. Furthermore, its aromatic framework allows for modification, enabling the tailoring of properties for specific applications. Despite its potential, challenges remain in translating the properties of N-(5-methoxy-2-phenoxyphenyl)methanesulfonamide into practical applications. Comprehensive studies on its toxicity, environmental impact, and long-term stability are necessary to advance its use in pharmaceuticals or materials science. The synthesis and evaluation of N-(5-methoxy-2-phenoxyphenyl)methanesulfonamide exemplify the innovative approaches in designing multifunctional molecules. Its structural attributes and biological potential underline the importance of continued research into sulfonamide derivatives, fostering advancements in both therapeutic and industrial domains. |
| Market Analysis Reports |
| List of Reports Available for N-(5-Methoxy-2-Phenoxyphenyl)methanesulfonamide |