Online Database of Chemicals from Around the World
| Shanghai Zhuyao Pharmaceutical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (021) 5859-6383 | |||
 |
marketing@pharmalego.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2023 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Organic raw materials >> Heterocyclic compound >> Piperidines |
|---|---|
| Name | Methyl 1-(5-bromopyridin-2-yl)piperidine-4-carboxylate |
| Molecular Structure | 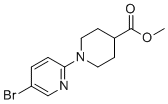 |
| Molecular Formula | C12H15BrN2O2 |
| Molecular Weight | 299.16 |
| CAS Registry Number | 1289027-00-4 |
| SMILES | COC(=O)C1CCN(CC1)C2=NC=C(C=C2)Br |
| Density | 1.4±0.1 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.563, Calc.* |
| Boiling Point | 389.5±42.0 ºC (760 mmHg), Calc.* |
| Flash Point | 189.4±27.9 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H315-H319-H335 Details |
| Precautionary Statements | P261-P305+351+338-P302+352 Details |
| SDS | Available |
|
Methyl 1-(5-bromopyridin-2-yl)piperidine-4-carboxylate is an organic compound that belongs to the class of piperidine derivatives, which are widely recognized for their pharmacological and synthetic importance. This chemical substance, characterized by the presence of a piperidine ring attached to a pyridine derivative, has attracted attention due to its potential use in medicinal chemistry and organic synthesis. The discovery of methyl 1-(5-bromopyridin-2-yl)piperidine-4-carboxylate stems from the growing interest in halogenated pyridine derivatives. Pyridine and its derivatives are known for their versatility in chemical reactions and interactions, making them valuable in a variety of chemical applications. The introduction of the bromine atom at the 5-position of the pyridine ring significantly enhances the reactivity of the molecule, particularly in its ability to undergo various nucleophilic substitution reactions. In terms of applications, methyl 1-(5-bromopyridin-2-yl)piperidine-4-carboxylate serves as an important intermediate in the synthesis of more complex molecules, particularly in the field of drug development. The combination of the piperidine ring and the halogenated pyridine unit provides a versatile structure that can be modified to produce a range of biologically active compounds. This makes it a valuable building block for the synthesis of potential therapeutic agents, especially those targeting neurological and cardiovascular diseases. Piperidine derivatives, such as methyl 1-(5-bromopyridin-2-yl)piperidine-4-carboxylate, are known for their ability to interact with various receptors in the central nervous system. The piperidine ring, with its nitrogen atom, is particularly useful in drug design as it can mimic or modulate the effects of neurotransmitters, making it an essential structure in the development of drugs for treating conditions like depression, anxiety, and schizophrenia. The halogen substitution on the pyridine ring can further influence the binding affinity of these compounds, making them more potent or selective in targeting specific receptors. In addition to its role in medicinal chemistry, methyl 1-(5-bromopyridin-2-yl)piperidine-4-carboxylate is also valuable in the field of organic synthesis. The presence of both the piperidine and pyridine rings provides a robust platform for the synthesis of complex organic molecules. This makes it useful in the production of agrochemicals, where pyridine derivatives are often utilized for the development of herbicides, fungicides, and insecticides. The bromine atom in the pyridine ring can enhance the compound's stability and reactivity, making it effective in such applications. Moreover, the compound is of interest in the field of materials science, particularly in the design of organic semiconductors and other electronic materials. The functional groups attached to the pyridine and piperidine rings can be tailored to improve the material’s electronic properties, making it useful for various electronic and optoelectronic applications. In summary, methyl 1-(5-bromopyridin-2-yl)piperidine-4-carboxylate is an important chemical compound with diverse applications in medicinal chemistry, organic synthesis, agrochemical development, and materials science. Its unique structure, which combines a piperidine ring with a halogenated pyridine unit, allows for its use in the development of biologically active molecules and advanced materials, making it a key player in modern chemical research and industry. |
| Market Analysis Reports |
| List of Reports Available for Methyl 1-(5-bromopyridin-2-yl)piperidine-4-carboxylate |