Online Database of Chemicals from Around the World
| Hangzhou Verychem Science And Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8816-2785 +86 13606544505 | |||
 |
lucy@verychem.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink massive supplier since 2021 | ||||
| Suzhou Rovathin Foreign Trade Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (512) 6581-6829 6197-8430 6063-5156 | |||
 |
sales@rovathin.com | |||
| Chemical distributor since 2007 | ||||
| chemBlink standard supplier since 2007 | ||||
| Sigma-Aldrich, Inc. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6141-5566 800-819-3336 | |||
 |
orderCN@sial.com | |||
| Chemical manufacturer since 1992 | ||||
| chemBlink standard supplier since 2013 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Classification | Biochemical >> Condensing agent |
|---|---|
| Name | Chlorotripyrrolidinophosphonium hexafluorophosphate |
| Synonyms | PyClOP |
| Molecular Structure | 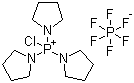 |
| Molecular Formula | C12H24ClN3P.PF6 |
| Molecular Weight | 421.73 |
| CAS Registry Number | 133894-48-1 |
| EC Number | 628-539-2 |
| SMILES | C1CCN(C1)[P+](N2CCCC2)(N3CCCC3)Cl.F[P-](F)(F)(F)(F)F |
| Melting point | 145 ºC |
|---|---|
| Hazard Symbols |
 GHS05 Danger Details
GHS05 Danger Details | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H314 Details | ||||||||||||||||||||
| Precautionary Statements | P260-P264-P280-P301+P330+P331-P302+P361+P354-P304+P340-P305+P354+P338-P316-P321-P363-P405-P501 Details | ||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||
| |||||||||||||||||||||
| Transport Information | UN 1759 | ||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||
|
Chlorotripyrrolidinephosphine hexafluorophosphate, commonly abbreviated as PyAOP, is a versatile coupling reagent widely used in peptide synthesis and organic chemistry. Chlorotripyrrolidinephosphine hexafluorophosphate was developed in the early 21st century as an improved alternative to existing peptide coupling reagents. Its synthesis involves the reaction of trispyrrolidinephosphine chloride with hexafluorophosphate, yielding a crystalline solid that is more soluble in organic solvents and stable under a wide range of reaction conditions. Chemically, PyAOP has a trispyrrolidinephosphine core and a hexafluorophosphate counterion. This structure gives PyAOP excellent nucleophilic and electrophilic properties, making it very effective in coupling reactions of amino acids or peptides. Its stability in protic and aprotic solvents ensures reliable performance in peptide synthesis without significant side reactions. PyAOP is primarily used as a coupling reagent in peptide synthesis due to its ability to form a stable amide bond between the carboxyl (-COOH) and amino (-NH2) groups of amino acids or peptides. The coupling reaction proceeds rapidly and selectively, yielding high-purity peptides suitable for pharmaceutical research, bioconjugate synthesis, and proteomics studies. In biochemistry and biotechnology, PyAOPs facilitate the conjugation of peptides or proteins to other molecules, such as fluorophores, drugs, or nanoparticles. This bioconjugation strategy enhances the functionality and specificity of biomolecules and can be used for targeted drug delivery, molecular imaging, and diagnostic assays. PyAOPs are compatible with solid-phase peptide synthesis methods, allowing for efficient elongation of peptides on solid supports. This compatibility simplifies the synthesis of long and complex peptides, oligonucleotides, and peptide nucleic acids (PNAs), which are critical for studying protein-protein interactions and designing therapeutics. Ongoing research explores advanced applications of PyAOPs in: designing peptide-based therapeutics with enhanced bioavailability, stability, and therapeutic efficacy for the treatment of diseases such as cancer, diabetes, and infectious diseases; exploiting the coupling efficiency and biocompatibility of PyAOPs to develop peptide-functionalized materials for biosensors, biomaterials, and tissue engineering applications; and investigating PyAOP-mediated chemoselective reactions and post-translational modifications to elucidate biological mechanisms and develop new tools for molecular biology research. Because PyAOPs are reactive and contact with chemicals can be a health hazard, they need to be handled under controlled laboratory conditions. Manufacturers adhere to strict safety protocols and regulatory guidelines to ensure safe handling, storage, and disposal in research and industrial settings. References 2006. Total Synthesis of Bioactive Peptides and Depsipeptides from Marine Opisthobranch Molluscs. Progress in Molecular and Subcellular Biology, 43. DOI: 10.1007/978-3-540-30880-5_15 2005. Phosphonium and Uronium/Guanidinium Salts. Science of Synthesis. URL: https://science-of-synthesis.thieme.com/app/text/?id=SD-021-00749 1993. NCA: A troublesome by-product in difficult amino acid coupling reactions. Peptides. DOI: 10.1007/978-94-011-1470-7_98 |
| Market Analysis Reports |
| List of Reports Available for Chlorotripyrrolidinophosphonium hexafluorophosphate |