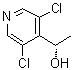
(alphaS)-3,5-Dichloro-alpha-methyl-4-pyridinemethanol is a chemical compound that has been of interest due to its structural features, which include a pyridine ring with both chloro and methyl substituents at specific positions. The compound belongs to the class of pyridine derivatives, which are known for their varied chemical and biological properties. Pyridine and its derivatives are often utilized in the synthesis of pharmaceuticals, agrochemicals, and materials, owing to their versatility and reactivity.
This specific compound, featuring a dichloro substitution pattern and a hydroxymethyl group, can be synthesized through methods that allow for selective chlorination and functionalization of the pyridine ring. The chirality at the alpha position of the methyl group is crucial, and the (alphaS) configuration can influence the compound's properties, particularly in terms of its interactions with biological systems or its behavior in chemical reactions.
The potential applications of (alphaS)-3,5-Dichloro-alpha-methyl-4-pyridinemethanol are significant in fields such as medicinal chemistry, where pyridine derivatives are commonly employed for their ability to bind to receptors or enzymes. The chloro and methyl groups could enhance the compound's pharmacokinetic properties, making it a candidate for further development as a bioactive molecule. Additionally, the hydroxymethyl group could provide a site for further functionalization, allowing for the design of more complex molecules with tailored properties.
In conclusion, (alphaS)-3,5-Dichloro-alpha-methyl-4-pyridinemethanol is an interesting compound with potential applications in various fields of chemistry, especially in the development of new pharmaceuticals or agrochemicals. Its synthesis and structural properties make it a valuable subject of study in the context of medicinal chemistry and materials science.
|





 GHS07 Warning Details
GHS07 Warning Details