Online Database of Chemicals from Around the World
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Zhejiang Chempharm Industry&trading Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8770-1568 | |||
 |
export@chempharm.cn | |||
| Chemical distributor since 1996 | ||||
| chemBlink standard supplier since 2007 | ||||
| Taizhou Tongxin Biopharmaceutical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 18652728585 | |||
 |
sales@allyrise.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2013 | ||||
| chemBlink standard supplier since 2013 | ||||
| Hangzhou Cherry Pharmaceutical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8163-6070 +86 18042403330 | |||
 |
info@cherrypharmatech.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2015 | ||||
| chemBlink standard supplier since 2015 | ||||
| Cangzhou Enke Pharma-tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (317) 510-5699 510-6597 +86 15533709196 | |||
 |
sale@enkepharma.com enkepharma@126.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
 |
WeChat: ymzhao | |||
| Chemical manufacturer since 2011 | ||||
| chemBlink standard supplier since 2016 | ||||
| Wonderchem Co., Limited | Hong Kong | Inquire | ||
|---|---|---|---|---|
 |
+852 8192-5218 | |||
 |
sales@wonder-chem.com | |||
| Chemical distributor since 2016 | ||||
| chemBlink standard supplier since 2017 | ||||
| Hangzhou Cyanochem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8522-0831 +86 17788583750 | |||
 |
info@cyanochem.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2017 | ||||
| chemBlink standard supplier since 2018 | ||||
| Shanghai Fuxin Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3130-0828 +86 18645121291 | |||
 |
contact@fuxinpharm.com | |||
| Chemical manufacturer since 2016 | ||||
| chemBlink standard supplier since 2018 | ||||
| Classification | Organic raw materials >> Organoboron compounds |
|---|---|
| Name | N-(Pyridin-2-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide |
| Molecular Structure | 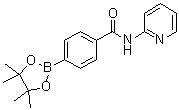 |
| Molecular Formula | C18H21BN2O3 |
| Molecular Weight | 324.18 |
| CAS Registry Number | 1383385-64-5 |
| EC Number | 817-663-4 |
| SMILES | B1(OC(C(O1)(C)C)(C)C)C2=CC=C(C=C2)C(=O)NC3=CC=CC=N3 |
| Density | 1.16±0.1 g/cm3 (20 ºC 760 Torr), Calc.* |
|---|---|
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (©1994-2016 ACD/Labs) |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P305+P351+P338 Details |
| SDS | Available |
|
N-(pyridin-2-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide is a complex organic compound that has been applied in various fields of chemical and biological research. Its structure combines a pyridine ring, a benzamide group, and a boronate ester, giving it unique properties that can be used in a variety of applications. The compound was developed through targeted synthetic efforts to combine the reactivity of boronate esters with the stability and functionality of benzamide derivatives. The introduction of a pyridine ring in the structure is intended to enhance the ability of the compound to interact with biological targets or act as a ligand in chemical reactions. The synthesis involves advanced organic chemistry techniques to create the desired functional groups and integrate them into a single molecular framework. In organic synthesis, the compound serves as a versatile building block for the creation of complex molecules. Its boronate ester group can participate in the Suzuki-Miyaura cross-coupling reaction, a powerful method for forming carbon-carbon bonds. This property makes it valuable in the synthesis of pharmaceuticals, agrochemicals, and advanced materials. The benzamide and pyridine moieties of the compound make it a candidate for drug discovery and development. Due to its ability to interact with a variety of biological targets, researchers are investigating its potential as a pharmaceutical agent. Its boronate group also provides opportunities to design inhibitors or modulators of specific enzymes or receptors. The boronate group in this compound can be used for molecular imaging. Its unique chemical properties can be used to develop imaging probes that can selectively bind to biological targets, helping to visualize specific cellular processes or disease states. This compound is being studied for its potential use as a catalyst in various chemical reactions. Its boronate functionality can enhance the reactivity of catalytic systems for the synthesis of complex organic compounds. |
| Market Analysis Reports |
| List of Reports Available for N-(Pyridin-2-yl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide |