Online Database of Chemicals from Around the World
| Guangzhou Topwork Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (20) 8731-7062 +86 13544492387 | |||
 |
sales@topworkchem.com topwork2004@hotmail.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
 |
WeChat: 13544492387 | |||
 |
WhatsApp: 13544492387 | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink standard supplier since 2006 | ||||
| Shaoyang Kerey Chemicals Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (739) 231-2559 +86 13807397282 | |||
 |
kereyinfo@126.com | |||
| Chemical manufacturer since 2005 | ||||
| chemBlink standard supplier since 2008 | ||||
| Taizhou Crene Biotechnology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (576) 8881-3233 8820-5808 +86 13396860566 | |||
 |
order@pharm-intermediates.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2011 | ||||
| chemBlink standard supplier since 2009 | ||||
| TCS Industry Limited | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (512) 5022-6427 | |||
 |
lsenfy@gmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Hubei Gedian Humanwell Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (27) 8759-7155 | |||
 |
market@steroid-chem.com | |||
| Chemical manufacturer since 2000 | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Hangzhou Utanpharma Biology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8682-1378 8682-0258 5683-6287 5683-6288 | |||
 |
sales@utanpharma.com utansale@hotmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Classification | API >> Hormone and endocrine-regulating drugs >> Prostaglandins |
|---|---|
| Name | Dutasteride |
| Synonyms | (5alpha,17beta)-N-{2,5-Bis(trifluoromethyl)phenyl}-3-oxo-4-azaandrost-l-ene-17-carboxamide |
| Molecular Structure | 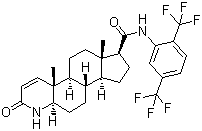 |
| Molecular Formula | C27H30F6N2O2 |
| Molecular Weight | 528.53 |
| CAS Registry Number | 164656-23-9 |
| EC Number | 638-758-5 |
| SMILES | C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2C(=O)NC4=C(C=CC(=C4)C(F)(F)F)C(F)(F)F)CC[C@@H]5[C@@]3(C=CC(=O)N5)C |
| Melting point | 242-250 ºC |
|---|---|
| Hazard Symbols |

 GHS07;GHS09 Danger Details
GHS07;GHS09 Danger Details | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H351-H360-H410-H412 Details | ||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P203-P273-P280-P318-P391-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||
|
Dutasteride, a compound with the chemical formula C_27H_30F_6N_2O_2, has had a major impact on the treatment of androgen-related diseases since its introduction. Known primarily for its effectiveness in treating benign prostatic hyperplasia (BPH) and androgenetic alopecia (male pattern baldness), dutasteride is a synthetic 5-alpha-reductase inhibitor designed to convert testosterone to dihydrotestosterone (DHT). The discovery of dutasteride stemmed from the need to find effective treatments for diseases caused by elevated levels of DHT, a potent androgen. In the late 1990s, pharmaceutical researchers discovered that inhibiting the 5-alpha-reductase enzyme that converts testosterone to DHT could mitigate the effects of this hormone. Developed by GlaxoSmithKline and approved by the FDA in 2001, dutasteride inhibits both type I and type II 5-alpha reductase isomers, providing a more comprehensive approach than earlier inhibitors such as finasteride, which only targeted type II. In clinical settings, dutasteride is most commonly used to treat benign prostatic hyperplasia (BPH). BPH is a noncancerous enlargement of the prostate that can cause urination problems in men. By reducing DHT levels, dutasteride can reduce the size of the prostate and relieve symptoms such as frequent urination, difficulty urinating, and a feeling of incomplete urination. Clinical trials have shown that dutasteride can significantly improve urine flow rate and reduce the need for BPH-related surgery. In addition to its use in the treatment of BPH, dutasteride has also been found to be effective in the treatment of androgenetic alopecia. Male baldness is largely due to the miniaturization of hair follicles caused by the effects of DHT. By reducing DHT levels in the scalp, dutasteride can help slow hair loss and promote hair regrowth. Studies have shown that dutasteride is more effective than finasteride in increasing hair volume and improving hair density, making it a valuable option for patients with hair loss. In addition, dutasteride is currently being investigated for its potential use in other conditions affected by androgens, such as hirsutism (excessive hair growth in women) and acne. Dutasteride's broad inhibitory effect on DHT synthesis suggests that it could provide therapeutic benefit for these conditions, although more research is needed to determine its effectiveness and safety in these conditions. Although dutasteride is generally well tolerated, it is not without side effects. Common adverse effects include decreased libido, erectile dysfunction, and ejaculation disorders, all of which are related to its mechanism of reducing DHT levels. These side effects are generally reversible after discontinuation of the drug. Another important consideration is that dutasteride may cause birth defects, so women who are pregnant or may become pregnant should avoid the drug. References 2024. The History of Hair Loss Treatments. Updates in Clinical Dermatology. DOI: 10.1007/978-3-031-74314-6_1 2013. Rationale for and Review of Neoadjuvant Therapy Prior to Radical Prostatectomy for Patients with High-Risk Prostate Cancer. Drugs, 73(13), 1417-1430. DOI: 10.1007/s40265-013-0107-2 2012. Dutasteride in localised prostate cancer management: the REDEEM randomised, double-blind, placebo-controlled trial. Lancet (London, England), 379(9821), 1103-1111. DOI: 10.1016/s0140-6736(11)61619-x |
| Market Analysis Reports |
| List of Reports Available for Dutasteride |