Online Database of Chemicals from Around the World
| Chizhou Dongsheng Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (576) 8429-2571 | |||
 |
ksz@dspharmchem.com | |||
| Chemical manufacturer since 1995 | ||||
| chemBlink premium supplier since 2011 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound |
|---|---|
| Name | 5-Fluoro-2-(4-(4-nitrophenyl)piperazin-1-yl)pyrimidine |
| Molecular Structure | 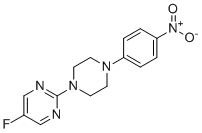 |
| Molecular Formula | C14H14FN5O2 |
| Molecular Weight | 303.29 |
| CAS Registry Number | 1928707-64-5 |
| SMILES | C1CN(CCN1C2=CC=C(C=C2)[N+](=O)[O-])C3=NC=C(C=N3)F |
|
5-Fluoro-2-(4-(4-nitrophenyl)piperazin-1-yl)pyrimidine is a significant chemical compound with noteworthy applications in medicinal chemistry. The molecule combines a fluorinated pyrimidine core with a piperazine moiety and a nitrophenyl group, contributing to its unique pharmacological properties. The compound was first synthesized and characterized as part of research aimed at developing new pharmaceuticals with enhanced biological activity. The introduction of fluorine into the pyrimidine ring increases the compound's metabolic stability and influences its binding affinity for biological targets. The piperazine ring provides additional sites for interaction with biological macromolecules, while the nitrophenyl group can affect the compound's electronic properties and solubility. In medicinal chemistry, 5-Fluoro-2-(4-(4-nitrophenyl)piperazin-1-yl)pyrimidine has been explored for its potential as a therapeutic agent. Its structure allows it to engage with specific biological targets, which is crucial for designing drugs that can modulate disease pathways effectively. The piperazine component enhances the compound's ability to interact with various receptors and enzymes, making it a candidate for developing treatments for neurological disorders and other conditions where piperazine-containing drugs have shown promise. Research has also demonstrated that this compound can function as a useful tool in drug discovery and development. Its unique structure enables it to serve as a lead compound in the development of new drugs. By modifying the core structure or substituents, researchers can explore a range of biological activities and optimize the compound for specific therapeutic applications. Additionally, 5-Fluoro-2-(4-(4-nitrophenyl)piperazin-1-yl)pyrimidine is utilized in the study of molecular mechanisms and in the development of diagnostic tools. Its ability to interact with specific biological targets makes it valuable for probing biological systems and understanding the role of particular receptors or enzymes in disease processes. The synthesis of 5-Fluoro-2-(4-(4-nitrophenyl)piperazin-1-yl)pyrimidine involves several key steps, including the introduction of the fluorine atom into the pyrimidine ring, the formation of the piperazine linkage, and the attachment of the nitrophenyl group. Advances in synthetic chemistry have facilitated the efficient production of this compound, enabling its use in both research and pharmaceutical applications. In summary, 5-Fluoro-2-(4-(4-nitrophenyl)piperazin-1-yl)pyrimidine exemplifies how chemical modifications can lead to the development of compounds with specific and useful biological activities. Its applications in drug discovery and development underscore the importance of structural chemistry in advancing medical science. |
| Market Analysis Reports |
| List of Reports Available for 5-Fluoro-2-(4-(4-nitrophenyl)piperazin-1-yl)pyrimidine |