Online Database of Chemicals from Around the World
| Futoh Chemicals Co., Ltd. | Japan | Inquire | ||
|---|---|---|---|---|
 |
+81 (52) 521-8171 | |||
 |
tokoro@futoh.co.jp | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Shanghai Shengnong International Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3468-9289 | |||
 |
yinlimei102@yahoo.com.cn | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2012 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Suzhou Candor Chemical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (512) 6262-6136 | |||
 |
julielu@candorchemi.com | |||
| Chemical distributor since 2016 | ||||
| chemBlink standard supplier since 2016 | ||||
| Hunan Yunbang Biomedical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (731) 8552-5705 +86 13319518603 | |||
 |
ivy@hnhbsj.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2014 | ||||
| chemBlink standard supplier since 2019 | ||||
| Classification | Organic raw materials >> Hydrocarbon compounds and their derivatives >> Hydrocarbon sulfonate |
|---|---|
| Name | alpha-Toluenesulfonyl chloride |
| Synonyms | Phenylmethanesulfonyl chloride; Benzylsulfonyl chloride |
| Molecular Structure | 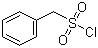 |
| Molecular Formula | C7H7ClO2S |
| Molecular Weight | 190.64 |
| CAS Registry Number | 1939-99-7 |
| EC Number | 217-717-1 |
| SMILES | C1=CC=C(C=C1)CS(=O)(=O)Cl |
| Melting point | 90-94 ºC |
|---|---|
| Boiling point | 120 ºC (10mm Hg), 278.9097 ºC (760mm Hg) |
| Refractive index | 1.624-1.626 |
| Water solubility | decomposes |
| Hazard Symbols |
 GHS05 Danger Details
GHS05 Danger Details | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H314 Details | ||||||||||||||||||||||||||||||||
| Precautionary Statements | P260-P264-P280-P301+P330+P331-P302+P361+P354-P304+P340-P305+P354+P338-P316-P321-P363-P405-P501 Details | ||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Transport Information | UN 3261 | ||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||
|
Alpha-Toluenesulfonyl chloride, also known as tosyl chloride, was first synthesized in the late 19th century as chemists explored the reactivity of toluene derivatives. Its discovery emerged from efforts to develop new chemical reagents and intermediates for organic synthesis. The synthesis process involves the chlorosulfonation of toluene, resulting in the formation of tosyl chloride. Alpha-Toluenesulfonyl chloride is widely used as a versatile reagent in organic synthesis. It serves as a precursor for the synthesis of various functionalized compounds, including sulfonamides, sulfonylureas, and sulfonate esters. Its ability to introduce the tosyl group (-SO2C6H4CH3) into organic molecules makes it valuable for modifying the reactivity and properties of target compounds. In organic synthesis, tosyl chloride is employed for protecting sensitive functional groups during chemical transformations. By converting reactive functional groups into less reactive tosyl derivatives, chemists can prevent unwanted side reactions and control the course of multi-step synthesis pathways. Additionally, tosyl chloride is used for activating nucleophiles and facilitating substitution reactions, such as the conversion of alcohols into tosylates for further transformations. Tosyl chloride finds application in peptide synthesis as a reagent for coupling amino acids and peptide fragments. It reacts with amino groups to form tosylamides, which serve as intermediates in peptide bond formation. This method, known as the Fmoc (9-fluorenylmethyloxycarbonyl) strategy, is widely used in solid-phase peptide synthesis. Tosyl chloride is utilized in the pharmaceutical industry for the synthesis of drug molecules and active pharmaceutical ingredients (APIs). Its role in introducing sulfonamide functional groups into drug candidates enhances their pharmacological properties, such as bioavailability, solubility, and metabolic stability. Tosyl derivatives of drug molecules may exhibit improved therapeutic efficacy or reduced toxicity compared to their parent compounds. In polymer chemistry, tosyl chloride is employed for functionalizing polymers and modifying their properties. It reacts with hydroxyl and amine groups on polymer chains to introduce tosyl groups, which can serve as reactive sites for further chemical modifications or cross-linking reactions. Tosyl-functionalized polymers find applications in areas such as coatings, adhesives, and biomaterials. References 2024. Recent Advances in the Synthesis of Acyclic Nucleosides and Their Therapeutic Applications. Topics in Current Chemistry, 382(3). DOI: 10.1007/s41061-024-00476-7 2024. Cyclolignan synthesis streamlined by enantioselective hydrogenation of tetrasubstituted olefins. Nature Synthesis, 3(6). DOI: 10.1038/s44160-024-00564-y 2023. Contemporary approaches to the synthesis of cyclic sulfonates (microreview). Chemistry of Heterocyclic Compounds, 59(11-12). DOI: 10.1007/s10593-024-03265-8 |
| Market Analysis Reports |
| List of Reports Available for alpha-Toluenesulfonyl chloride |