Online Database of Chemicals from Around the World
| Shandong Benrite New Chemical Materials Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (0533) 286-6658 | |||
 |
sales@benritechem.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
 |
WeChat: benritechem | |||
 |
WhatsApp: +8613021512891 | |||
| Chemical distributor since 2024 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Inorganic chemical industry >> Inorganic salt >> Carbides and carbonates |
|---|---|
| Name | 2-Butyne-1,4-diyl dimethyl biscarbonate |
| Synonyms | C,Ca(2)-2-Butyne-1,4-diyl bis(C-methyl carbonate); 4-methoxycarbonyloxybut-2-ynyl methyl carbonate |
| Molecular Structure | 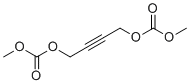 |
| Molecular Formula | C8H10O6 |
| Molecular Weight | 202.16 |
| CAS Registry Number | 197244-15-8 |
| SMILES | COC(=O)OCC#CCOC(=O)OC |
| Density | 1.2±0.1 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.452, Calc.* |
| Boiling Point | 260.1±25.0 ºC (760 mmHg), Calc.* |
| Flash Point | 109.5±23.2 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
|
2-Butyne-1,4-diyl dimethyl biscarbonate is a versatile compound characterized by its central alkyne functional group flanked by two carbonate moieties. This molecule is particularly notable for its dual reactivity, making it useful in organic synthesis and materials chemistry. The combination of an alkyne linker and reactive carbonate groups allows it to serve as a crosslinking agent, protecting group, or intermediate in the production of advanced polymeric materials and pharmaceuticals. The compound was first explored during investigations into functionalized alkynes and their ability to undergo selective transformations. Researchers sought molecules that could incorporate both the rigidity of an alkyne and the electrophilicity of carbonate groups. By coupling these structural features, chemists synthesized 2-butyne-1,4-diyl dimethyl biscarbonate to enhance the versatility of carbonates in organic synthesis. Its symmetrical structure facilitates predictable reactivity, which is useful in controlled polymerizations and functional group protection strategies. The synthesis of 2-butyne-1,4-diyl dimethyl biscarbonate typically involves the reaction of 2-butyne-1,4-diol with methyl chloroformate under basic conditions. This method ensures that both hydroxyl groups on the diol are converted to carbonate esters, forming a stable product. The alkyne functional group remains intact throughout the reaction, allowing for further functionalization or coupling reactions in subsequent steps. This compound finds applications in several key areas. One notable application is in the synthesis of polycarbonates and polyesters where it acts as a bifunctional monomer. The presence of the alkyne spacer allows for the introduction of rigidity and controlled spacing between polymer chains. This property is particularly valuable in the development of high-performance materials with enhanced thermal and mechanical properties. In organic synthesis, 2-butyne-1,4-diyl dimethyl biscarbonate is used as a protecting group for diols and alcohols. The carbonate groups protect hydroxyl functionalities during multi-step reactions, and their removal can be achieved under mild conditions, regenerating the original hydroxyl groups. The alkyne functionality enables further transformations, such as click chemistry or cross-coupling reactions, which broaden the scope of its synthetic utility. Additionally, the compound has potential in pharmaceutical development. Its structure can be used to build molecular scaffolds that incorporate both rigid and flexible elements. These scaffolds are useful in designing molecules with improved bioactivity and pharmacokinetic properties. Researchers are also investigating its utility in drug delivery systems where controlled release and functional group stability are critical. Overall, 2-butyne-1,4-diyl dimethyl biscarbonate serves as a valuable tool in materials science, synthetic chemistry, and pharmaceutical research due to its unique combination of alkyne and carbonate functionalities. |
| Market Analysis Reports |
| List of Reports Available for 2-Butyne-1,4-diyl dimethyl biscarbonate |