Online Database of Chemicals from Around the World
| Fenhe Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (021) 3392-6068 | |||
 |
julius.wei@fenhechem.com | |||
| Chemical manufacturer since 1997 | ||||
| chemBlink standard supplier since 2024 | ||||
| Name | 4,5-Dichloro-2-methyl-1H-benzimidazole |
|---|---|
| Molecular Structure | 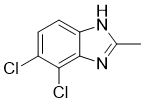 |
| Molecular Formula | C8H6Cl2N2 |
| Molecular Weight | 201.05 |
| CAS Registry Number | 20039-29-6 |
| SMILES | Cc1[nH]c2ccc(c(c2n1)Cl)Cl |
| Density | 1.5±0.1 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.682, Calc.* |
| Boiling Point | 409.0±25.0 ºC (760 mmHg), Calc.* |
| Flash Point | 233.6±8.8 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
|
4,5-Dichloro-2-methyl-1H-benzimidazole is a chlorinated benzimidazole derivative with the molecular formula C8H6Cl2N2. This compound, characterized by the presence of chlorine atoms at the 4 and 5 positions and a methyl group at the 2 position of the benzimidazole ring, has gained attention due to its unique chemical properties and potential applications in various fields. The discovery of 4,5-Dichloro-2-methyl-1H-benzimidazole is rooted in the broader exploration of benzimidazole derivatives, which began in the early 20th century. Benzimidazoles were initially studied for their biological activity, particularly in the context of antifungal, antiparasitic, and antimicrobial agents. Researchers discovered that introducing halogen atoms, such as chlorine, into the benzimidazole structure could enhance its stability and biological activity, leading to the development of new compounds with improved pharmacological profiles. In terms of application, 4,5-Dichloro-2-methyl-1H-benzimidazole is primarily used in pharmaceutical research and development. Its structure serves as a key building block in the synthesis of various therapeutic agents, particularly those targeting diseases where benzimidazole derivatives have shown efficacy. The presence of chlorine atoms in the molecule is believed to enhance its interaction with biological targets, making it a valuable component in drug design. One significant application of 4,5-Dichloro-2-methyl-1H-benzimidazole is in the development of kinase inhibitors. Kinase inhibitors are a class of drugs that block the activity of kinases—enzymes that play a critical role in cell signaling pathways involved in cancer and other diseases. The dichloro-substituted benzimidazole scaffold is particularly attractive for designing kinase inhibitors due to its ability to fit into the active sites of these enzymes, thereby interfering with their function and potentially halting disease progression. In addition to its use in pharmaceutical research, 4,5-Dichloro-2-methyl-1H-benzimidazole is also employed in organic synthesis as a versatile intermediate. Its chlorinated structure allows for further chemical modifications, making it a valuable starting material in the preparation of more complex molecules. This flexibility is essential in the development of novel compounds with specific properties, whether for medicinal chemistry or materials science. The compound’s chemical stability and reactivity also make it a useful tool in the study of benzimidazole derivatives. Researchers utilize 4,5-Dichloro-2-methyl-1H-benzimidazole as a model compound to investigate the effects of chlorine substitution on the chemical and physical properties of benzimidazoles. These studies contribute to a deeper understanding of the structure-activity relationships that govern the behavior of these compounds, which is critical for the rational design of new drugs and materials. Despite its potential benefits, 4,5-Dichloro-2-methyl-1H-benzimidazole, like many chlorinated organic compounds, must be handled with care due to its potential health and environmental risks. It is classified as hazardous, with possible risks of skin and respiratory irritation upon exposure. Therefore, proper safety measures, including the use of personal protective equipment and adherence to safe disposal practices, are essential when working with this compound. In conclusion, 4,5-Dichloro-2-methyl-1H-benzimidazole is a significant compound in pharmaceutical research and organic synthesis. Its discovery and subsequent application highlight the importance of halogenated benzimidazole derivatives in drug development and the synthesis of complex organic molecules. The compound's unique chemical properties make it a valuable tool in the ongoing exploration of new therapeutic agents and advanced materials. |
| Market Analysis Reports |
| List of Reports Available for 4,5-Dichloro-2-methyl-1H-benzimidazole |