Online Database of Chemicals from Around the World
| Yancheng Jiangzhong Chemicals Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (515) 8898-1987 +86 13365196093 +8613918567443 | |||
 |
richard.li_jzchem@263.net | |||
 |
QQ chat | |||
| Chemical manufacturer since 1989 | ||||
| chemBlink standard supplier since 2011 | ||||
| Hangzhou Chuan Shuo Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8760-9021 | |||
 |
qian@transol.cn | |||
| Chemical manufacturer since 2017 | ||||
| chemBlink standard supplier since 2025 | ||||
| Achemica | Switzerland | Inquire | ||
|---|---|---|---|---|
 |
+41 (24) 466-2929 | |||
 |
contact@achemica.com | |||
| Chemical manufacturer since 2010 | ||||
| Classification | Organic raw materials >> Carboxylic compounds and derivatives >> Halogenation, sulfonation, nitration or nitrosation of carboxylic acids |
|---|---|
| Name | 2-Chloro-6-mercaptobenzoic acid |
| Synonyms | 6-Chloro-2-mercaptobenzoic acid |
| Molecular Structure | 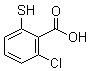 |
| Molecular Formula | C7H5ClO2S |
| Molecular Weight | 188.63 |
| CAS Registry Number | 20324-51-0 |
| SMILES | C1=CC(=C(C(=C1)Cl)C(=O)O)S |
| Density | 1.5±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 323.2±27.0 ºC 760 mmHg (Calc.)* |
| Flash point | 149.2±23.7 ºC (Calc.)* |
| Index of refraction | 1.652 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H315-H319 Details |
| Precautionary Statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 Details |
| SDS | Available |
|
2-Chloro-6-mercaptobenzoic acid is an aromatic carboxylic acid derivative bearing both a chlorine atom and a thiol group on a benzene ring substituted with a carboxylic acid. Its molecular formula is C7H5ClO2S. The structure features a carboxylic acid (–COOH) at the 1-position, a chlorine atom (–Cl) at the 2-position (ortho to the carboxylic acid), and a thiol group (–SH) at the 6-position (meta to the carboxyl group and ortho to the chlorine). This combination of functional groups allows the molecule to participate in a variety of chemical reactions, making it useful as a building block in organic synthesis and coordination chemistry. The compound is typically prepared via directed substitution reactions on a pre-functionalized benzoic acid framework. One common route involves chlorination of 6-mercaptobenzoic acid using reagents such as thionyl chloride or phosphorus pentachloride, which preferentially introduces a chlorine atom at the 2-position due to the directing effects of the carboxylic and thiol groups. Alternatively, thiolation of 2-chlorobenzoic acid derivatives using thiourea or other sulfur sources can yield the desired product after appropriate hydrolysis and purification steps. 2-Chloro-6-mercaptobenzoic acid has been studied primarily as an intermediate in the synthesis of more complex molecules. The presence of both a thiol and carboxylic acid group allows it to act as a bidentate ligand in coordination chemistry. It can chelate metal centers such as palladium, copper, and gold, forming stable complexes that have potential uses in catalysis, sensing, or materials science. These complexes often exhibit interesting spectroscopic or catalytic properties due to the electron-donating nature of the thiol group and the electron-withdrawing character of the carboxylic acid and chloro substituents. In organic synthesis, the thiol group provides a reactive site for derivatization. It can be oxidized to disulfides, or used in thiol–ene reactions, nucleophilic substitution, or cross-coupling processes. The chlorine atom at the 2-position also offers an electrophilic center that can undergo substitution by nucleophiles such as amines, alcohols, or thiols, allowing the molecule to serve as a versatile intermediate for the synthesis of heterocycles, peptides, or functionalized benzoic acid derivatives. The carboxylic acid group provides solubility in polar solvents and allows coupling with alcohols or amines to form esters or amides, respectively. This functional diversity makes 2-chloro-6-mercaptobenzoic acid a valuable component in medicinal chemistry research, especially in the development of compounds with metal-binding, antioxidant, or enzyme-inhibitory properties. Although the compound itself is not widely known as a pharmaceutical agent, derivatives or metal complexes based on its structure have been investigated for biological activity. In analytical chemistry, this compound or its derivatives have occasionally been used in spectrophotometric assays or as modifiers in chromatography, particularly when interactions with metal ions are relevant. The presence of both hydrophilic and hydrophobic functional groups enables interaction with various phases and analytes. From a safety and handling perspective, 2-chloro-6-mercaptobenzoic acid should be stored in a cool, dry place in tightly sealed containers to prevent oxidation of the thiol group. The compound may release unpleasant odors typical of thiols and should be handled in a well-ventilated area. As with many thiol-containing compounds, it may pose irritant or sensitization risks, and standard laboratory protective equipment such as gloves, goggles, and lab coats is recommended. In summary, 2-chloro-6-mercaptobenzoic acid is a functionalized aromatic compound that serves as a useful intermediate in synthetic chemistry, particularly in the formation of coordination complexes and functional organic molecules. Its multiple reactive sites provide opportunities for derivatization and incorporation into more complex structures in research involving catalysis, materials, and bioactive compounds. |
| Market Analysis Reports |
| List of Reports Available for 2-Chloro-6-mercaptobenzoic acid |