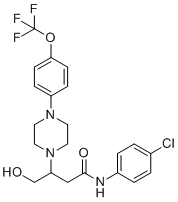
VBIT-4 is a small-molecule compound known for its role as a selective inhibitor of the Voltage-Dependent Anion Channel 1 (VDAC1), a mitochondrial outer membrane protein that plays a key role in regulating mitochondrial metabolism, apoptosis, and cell survival. VDAC1 functions as a gatekeeper of the mitochondria, facilitating the transport of ions and metabolites, including ATP and other respiratory substrates, between the mitochondria and the cytosol. Dysregulation of VDAC1 has been implicated in various pathological conditions, including cancer, neurodegeneration, and metabolic disorders. VBIT-4 (VDAC1-based Inhibitory Tool 4) was developed as part of a research effort to identify small molecules capable of modulating VDAC1 activity without inducing mitochondrial dysfunction or general cytotoxicity.
The compound was discovered through a structure-based drug design and screening process aimed at finding molecules that bind VDAC1 and prevent its pro-apoptotic conformational changes. One of the critical features of VBIT-4 is its ability to inhibit the interaction between VDAC1 and pro-apoptotic proteins such as Bax, thereby blocking mitochondrial outer membrane permeabilization (MOMP), a key event in the intrinsic pathway of apoptosis. By stabilizing VDAC1 in a non-conducting conformation, VBIT-4 prevents the release of cytochrome c and other apoptogenic factors into the cytosol, which in turn halts the downstream activation of caspases and apoptotic cell death.
The development of VBIT-4 was motivated by the need to explore therapeutic strategies for diseases involving excessive apoptosis, such as certain neurodegenerative conditions, as well as disorders characterized by mitochondrial dysfunction. Preclinical studies have demonstrated that VBIT-4 can protect neuronal cells from oxidative stress-induced apoptosis, highlighting its potential in the treatment of diseases like Alzheimer's disease and Parkinson’s disease. In models of neurodegeneration, treatment with VBIT-4 reduced the accumulation of damaged mitochondria and improved cellular bioenergetics, suggesting that its protective effects are mediated through both direct inhibition of VDAC1 and indirect support of mitochondrial integrity.
In oncology research, VBIT-4 has been explored as a tool to study VDAC1’s contribution to cancer cell metabolism and survival. Cancer cells often exhibit upregulated VDAC1 expression, contributing to increased mitochondrial metabolic flux and resistance to cell death. VBIT-4 has been used experimentally to disrupt these processes, sensitizing tumor cells to chemotherapeutic agents or inducing energy stress. However, its primary value in cancer studies has been as a chemical probe rather than as a candidate for monotherapy, due to the complexity of VDAC1’s role in both cell survival and death.
Mechanistically, VBIT-4 does not block the VDAC1 pore completely but modulates its conductance and protein-protein interactions, which differentiates it from traditional channel blockers. This selectivity allows for the maintenance of basic mitochondrial function while modulating pathological signaling pathways. VBIT-4 has shown minimal off-target activity in cell-based assays and retains stability under physiological conditions, making it a suitable candidate for in vivo pharmacological studies in animal models.
VBIT-4 is primarily utilized in academic and preclinical research settings, where it serves as a molecular tool to dissect the functions of VDAC1 in various cellular contexts. Its pharmacokinetic properties and lack of systemic toxicity in tested models have enabled its application in long-term studies of mitochondrial regulation. However, further work is required to optimize its drug-like properties for potential therapeutic development, particularly with regard to tissue targeting, oral bioavailability, and metabolic stability. The insights gained from studies using VBIT-4 continue to advance the understanding of mitochondrial biology and its relevance to disease pathogenesis.
|


 GHS07 Warning Details
GHS07 Warning Details