Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Hubei Teyer Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (728) 533-2622 | |||
 |
info@hubeiteyer.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2025 | ||||
| Zhangjiagang Xinyi Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (512) 5866-7398 5859-1449 | |||
 |
zjgxyhg@126.com | |||
| Chemical manufacturer | ||||
| Interchim | France | Inquire | ||
|---|---|---|---|---|
 |
+33 (4) 7003-7301 | |||
 |
interfine@mail.interchim.fr | |||
| Chemical manufacturer | ||||
| Classification | Chemical reagent >> Organic reagent >> Sulfonate / sulfinate |
|---|---|
| Name | Sodium (+)-10-camphorsulfonate |
| Synonyms | D-Camphor-10-sulfonic acid sodium salt |
| Molecular Structure | 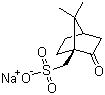 |
| Molecular Formula | C10H15NaO4S |
| Molecular Weight | 254.28 |
| CAS Registry Number | 21791-94-6 |
| EC Number | 244-581-0 |
| SMILES | CC1([C@@H]2CC[C@]1(C(=O)C2)CS(=O)(=O)[O-])C.[Na+] |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P305+P351+P338 Details |
| SDS | Available |
|
Sodium (+)-10-camphorsulfonate is a chemical compound that is widely used in various fields of chemistry, particularly in the areas of asymmetric synthesis and catalysis. This compound is derived from camphor, a naturally occurring terpene that has a strong, characteristic odor. The sodium salt form of 10-camphorsulfonic acid is commonly employed as a chiral auxiliary and a reagent in synthetic organic chemistry due to its ability to induce chirality in reactions. The discovery of camphorsulfonic acid and its salts can be traced back to the mid-20th century, when researchers began exploring the use of chiral substances in stereoselective and enantioselective synthesis. Camphorsulfonic acid itself is a versatile compound, but its sodium salt form, sodium (+)-10-camphorsulfonate, is particularly notable for its ability to facilitate asymmetric reactions by influencing the stereochemistry of the products. Sodium (+)-10-camphorsulfonate is often used as a chiral ion pair in the resolution of racemic mixtures, which are mixtures containing equal amounts of enantiomers. In the field of asymmetric synthesis, it serves as a chiral auxiliary in reactions such as alkylations and reductions, where it helps to direct the formation of a specific enantiomer over the other. By providing a chiral environment for the reaction, this compound ensures that the desired stereoisomer is produced with high selectivity. In addition to its applications in asymmetric synthesis, sodium (+)-10-camphorsulfonate has been employed as a reagent in catalysis, particularly in processes that require chiral catalysts. It has been used in the development of chiral catalysts for a variety of reactions, including enantioselective reactions involving the formation of carbon-carbon bonds. The compound’s ability to stabilize certain reaction intermediates and to influence the stereochemistry of the transition state makes it a valuable tool in catalytic processes. Furthermore, sodium (+)-10-camphorsulfonate is utilized in the preparation of other chiral derivatives and compounds, which are important in the synthesis of pharmaceuticals, agrochemicals, and other fine chemicals. Its role in promoting chirality in organic synthesis has made it an indispensable reagent in the production of optically active compounds, which are crucial in industries such as drug development and chemical manufacturing. The compound is typically used in small quantities as part of a reaction mixture or in the preparation of chiral reagents. While it is generally considered to be a safe and useful reagent, it is important to handle it with care, as with any chemical substance, and to follow appropriate safety guidelines when working with it in the laboratory. In conclusion, sodium (+)-10-camphorsulfonate is a valuable chemical in the field of synthetic organic chemistry, particularly in asymmetric synthesis and catalysis. Its ability to influence stereochemistry and facilitate the production of chiral compounds has made it an essential tool for researchers and chemists working in the area of chiral synthesis. Its applications in the preparation of optically active compounds have significant importance in the pharmaceutical and chemical industries. References 2008. Reaction of In Situ Generated Sulfonyl Halides with Amines. Science of Synthesis |
| Market Analysis Reports |
| List of Reports Available for Sodium (+)-10-camphorsulfonate |