Online Database of Chemicals from Around the World
| MolScanner | Singapore | Inquire | ||
|---|---|---|---|---|
 |
+86 18621675448 | |||
 |
marketing@molscanner.com | |||
 |
WhatsApp: 9896 7603 | |||
| Chemical manufacturer since 2025 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyridazine |
|---|---|
| Name | Lithium 4,6-Dichloropyridazine-3-carboxylate |
| Molecular Structure | 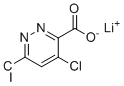 |
| Molecular Formula | C5HCl2LiN2O2 |
| Molecular Weight | 198.92 |
| CAS Registry Number | 2245238-80-4 |
| EC Number | 946-164-5 |
| SMILES | [Li+].C1=C(C(=NN=C1Cl)C(=O)[O-])Cl |
| Hazard Symbols |

 GHS05;GHS07 Danger Details
GHS05;GHS07 Danger Details |
|---|---|
| Hazard Statements | H302-H312-H315-H317-H318-H319-H332-H335 Details |
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P272-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P305+P354+P338-P317-P319-P321-P330-P332+P317-P333+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details |
| SDS | Available |
|
Lithium 4,6-dichloropyridazine-3-carboxylate is a synthetic organic lithium salt derived from the pyridazine heterocyclic system, which is characterized by a six-membered aromatic ring containing two adjacent nitrogen atoms at positions 1 and 2. This compound includes chlorine atoms at the 4- and 6-positions of the ring and a carboxylate group at the 3-position that is ionically associated with a lithium cation. It is generally prepared by neutralizing 4,6-dichloropyridazine-3-carboxylic acid with lithium hydroxide under aqueous or alcoholic conditions. The pyridazine ring system was first synthesized and characterized in the late 19th and early 20th centuries, but it was not until the mid-20th century that the potential applications of substituted pyridazines in pharmaceutical and agrochemical research became widely appreciated. Substituents like chloro and carboxylate groups increase the reactivity and versatility of the pyridazine ring, particularly in substitution reactions involving nucleophiles. The chlorine atoms at the 4- and 6-positions are reactive sites that can undergo displacement under mild conditions, enabling the synthesis of a wide variety of derivatives. Lithium 4,6-dichloropyridazine-3-carboxylate is primarily employed as an intermediate in the synthesis of more complex organic molecules. It serves as a valuable precursor for the development of pharmaceutical candidates and agrochemicals. In drug discovery, pyridazine derivatives have been studied for their activities as enzyme inhibitors, receptor modulators, and anti-inflammatory agents. The dichlorinated pyridazine ring provides a scaffold for the development of compounds targeting specific biological pathways, including kinases and other regulatory proteins. In agrochemical applications, substituted pyridazines have been investigated for herbicidal, fungicidal, and insecticidal properties. The carboxylate group in lithium 4,6-dichloropyridazine-3-carboxylate can be esterified or converted into amides, which are structural motifs commonly found in bioactive molecules. Additionally, the chlorine atoms facilitate further functionalization, allowing researchers to build structurally diverse libraries for structure-activity relationship studies. The lithium salt form enhances the compound's solubility in polar solvents and can improve its reactivity in synthetic procedures. Compared to other alkali metal salts, lithium salts are often preferred in organic synthesis due to their smaller ionic radius and higher charge density, which can influence reaction rates and selectivities. In some cases, lithium salts are used to facilitate metalation or coordination with other reagents in multi-step synthesis routes. Overall, lithium 4,6-dichloropyridazine-3-carboxylate is a versatile and well-characterized intermediate that plays a critical role in the preparation of substituted heterocyclic compounds. Its applications span both pharmaceutical and agrochemical development, making it a compound of ongoing interest in synthetic and medicinal chemistry. References 2022. Synthesis of Deucravacitinib. Synfacts, 18(8). DOI: 10.1055/s-0041-1738228 |
| Market Analysis Reports |
| List of Reports Available for Lithium 4,6-Dichloropyridazine-3-carboxylate |