Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Chemical reagent >> Organic reagent >> Sulfonate / sulfinate |
|---|---|
| Name | Pyridinium toluene-4-sulphonate |
| Synonyms | Pyridinium p-toluenesulfonate |
| Molecular Structure | 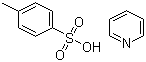 |
| Molecular Formula | C7H8O3S.C5H5N |
| Molecular Weight | 251.30 |
| CAS Registry Number | 24057-28-1 |
| EC Number | 246-002-7 |
| SMILES | CC1=CC=C(C=C1)S(=O)(=O)[O-].C1=CC=[NH+]C=C1 |
| Melting point | 116-120 ºC |
|---|---|
| Water solubility | decomposes |
| Hazard Symbols |

 GHS07;GHS08 Warning Details
GHS07;GHS08 Warning Details | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335-H373-H412 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P260-P261-P264-P264+P265-P271-P273-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||
|
Pyridinium toluene-4-sulphonate is an organic salt formed by the protonation of pyridine with p-toluenesulfonic acid, resulting in the pyridinium cation and the toluene-4-sulphonate anion. The compound is commonly represented by the formula \[C5H5NH]+\[CH3C6H4SO3]−, where the pyridine nitrogen carries a positive charge due to protonation, and the sulfonate group is the conjugate base of p-toluenesulfonic acid. Pyridinium toluene-4-sulphonate is typically prepared by mixing equimolar amounts of pyridine and p-toluenesulfonic acid in an appropriate solvent, such as ethanol or water, leading to salt formation through acid-base reaction. The product usually crystallizes as colorless or pale solids that are soluble in polar solvents. The salt serves as a mild acid catalyst in various organic synthesis reactions. Its acid strength derives from the p-toluenesulfonic acid component, while the pyridinium cation stabilizes the salt structure. Compared to free p-toluenesulfonic acid, this pyridinium salt offers enhanced handling convenience, reduced corrosiveness, and improved solubility properties in certain organic solvents. In synthetic chemistry, pyridinium toluene-4-sulphonate is applied as a catalyst for esterification, acetalization, and other acid-catalyzed transformations where controlled acidity is required. It facilitates reaction progress while minimizing side reactions and substrate degradation commonly associated with stronger acids. The compound is also used as a reagent or intermediate in the preparation of other organic salts and ionic liquids, benefiting from its stable crystalline nature and well-defined composition. Its ionic character allows incorporation into reaction media where acid catalysis without excess free acid is desired. Handling of pyridinium toluene-4-sulphonate is generally safe under normal laboratory conditions, but as an acidic salt, it may cause irritation to skin, eyes, and respiratory tract upon contact or inhalation. Proper personal protective equipment and ventilation are recommended. Thermal analysis indicates that the salt is stable under moderate heating, decomposing at elevated temperatures with the release of sulfonic acid derivatives and pyridine. This property is considered when using the compound in reactions requiring elevated temperatures. In summary, pyridinium toluene-4-sulphonate is an organic acid salt formed by protonated pyridine and toluene-4-sulphonate anion. It functions primarily as a mild acid catalyst in organic synthesis, offering advantages in handling and solubility over free p-toluenesulfonic acid. Its stability and defined acidity make it a useful reagent in various chemical transformations and preparative procedures. References 2024. Ring opening of epoxides: a facile approach towards the synthesis of polyketides and related stereoenriched natural products: a review. Molecular Diversity, 28(6). DOI: 10.1007/s11030-024-11057-7 2024. Synthesis of N-(iminyl)pyridinium salts from hydrazones by the Zincke reaction. Russian Chemical Bulletin, 73(5). DOI: 10.1007/s11172-024-4246-2 2024. Exploring the decadal evolution of indolizine scaffold for anticancer innovations: a comprehensive analysis. Medicinal Chemistry Research, 33(7). DOI: 10.1007/s00044-024-03280-6 |
| Market Analysis Reports |
| List of Reports Available for Pyridinium toluene-4-sulphonate |