Online Database of Chemicals from Around the World
| Auben Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 19121901603 | |||
 |
sales@aubenchem.com | |||
| Chemical distributor since 2023 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Carboxylic compounds and derivatives >> Carboxylic esters and their derivatives |
|---|---|
| Name | Benzyl (2R,3S,5R)-2-(hydroxymethyl)-3-((4-methoxyphenyl)(methyl)amino)-5-methylpyrrolidine-1-carboxylate |
| Molecular Structure | 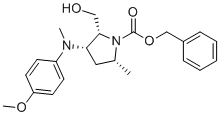 |
| Molecular Formula | C22H28N2O4 |
| Molecular Weight | 384.47 |
| CAS Registry Number | 2470016-18-1 |
| SMILES | O=C(N1[C@@H](CO)[C@@H](N(C2=CC=C(OC)C=C2)C)C[C@H]1C)OCC3=CC=CC=C3 |
| Density | 1.2±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 547.0±50.0 ºC 760 mmHg (Calc.)* |
| Flash point | 284.6±30.1 ºC (Calc.)* |
| Index of refraction | 1.6 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
|
Benzyl (2R,3S,5R)-2-(hydroxymethyl)-3-((4-methoxyphenyl)(methyl)amino)-5-methylpyrrolidine-1-carboxylate is a chiral pyrrolidine derivative reported in patent literature as a chemical intermediate used in the construction of small molecules targeting orexin receptors. Orexin receptors are G-protein-coupled receptors that play a key role in regulating sleep and wakefulness. Modulation of these receptors has been actively explored for therapeutic applications in sleep disorders, including narcolepsy and other hypersomnias. The design of small molecules for orexin receptors requires precise stereochemistry and functional group placement to ensure receptor specificity and pharmacological activity. The compound contains multiple functional groups that make it valuable in synthetic chemistry. The nitrogen of the pyrrolidine ring is protected with a benzyloxycarbonyl (Cbz) group, providing stability during chemical transformations. A hydroxymethyl group is present at the 2-position, a 4-methoxyphenylmethylamino substituent at the 3-position, and a methyl group at the 5-position. These structural features allow the compound to participate in stereoselective reactions and to retain defined three-dimensional geometry, which is crucial for the biological activity of the final molecules in orexin receptor programs. The stereochemistry of the compound, with 2R,3S,5R configuration, ensures that downstream transformations preserve the desired spatial arrangement required for receptor binding. In medicinal chemistry, such stereochemical control is essential because receptor interactions are highly dependent on the orientation of functional groups. Compounds with incorrect stereochemistry may exhibit reduced potency or altered selectivity, making the control of chiral centers a central consideration in drug design. This pyrrolidine derivative is synthesized through established organic chemistry methodologies that combine reductive amination, protection strategies, and purification steps to obtain the desired stereoisomer. The presence of the Cbz group allows selective functionalization of the nitrogen while maintaining the integrity of the pyrrolidine ring. The hydroxymethyl group is compatible with common nucleophilic reactions, and the 4-methoxyphenylmethylamino substituent provides both steric and electronic properties that can influence subsequent chemical transformations. Although the compound itself is not evaluated for direct pharmacological activity, its role in the synthesis of bioactive molecules targeting orexin receptors is well documented. Small molecule agonists of orexin receptors are designed to engage receptor binding pockets with high specificity. The pyrrolidine scaffold provides rigidity and spatial definition, while the attached functional groups contribute to hydrogen bonding, hydrophobic interactions, and overall molecular recognition. These characteristics are essential for achieving the desired in vitro and in vivo efficacy of orexin receptor modulators. Applications of molecules derived from this compound include research into central nervous system disorders and potential therapeutic development for sleep regulation. The structure has been utilized in the preparation of dual orexin receptor agonists and selective orexin 2 receptor agonists. Studies of these compounds demonstrate their ability to activate orexin signaling pathways and influence wakefulness in animal models. The pyrrolidine scaffold, including the benzyl and methoxyphenyl substituents, is a recurring motif in medicinal chemistry programs for central nervous system targets due to its favorable pharmacokinetic and pharmacodynamic properties. The discovery and use of benzyl (2R,3S,5R)-2-(hydroxymethyl)-3-((4-methoxyphenyl)(methyl)amino)-5-methylpyrrolidine-1-carboxylate illustrate the integration of stereoselective synthesis, functional group manipulation, and medicinal chemistry design principles. Its chemical properties, including stereochemistry, protection groups, and substituent patterns, have enabled the development of small molecules capable of modulating orexin receptor activity in a controlled and predictable manner. The compound remains a valuable chemical for research and development in sleep disorder therapeutics. References Dehui Zhang, David A. Perrey, Ann M. Decker, et al. (2021) Discovery of arylsulfonamides as dual orexin receptor agonists. Journal of Medicinal Chemistry 64(12) 8806–8825 DOI: 10.1021/acs.jmedchem.1c00841 Design and synthesis of novel orexin 2 receptor agonists with a 1,3,5-trioxazatriquinane skeleton. Bioorganic & Medicinal Chemistry Letters (2023) DOI: 10.1016/j.bmcl.2023.129151 |
| Market Analysis Reports |
| List of Reports Available for Benzyl (2R,3S,5R)-2-(hydroxymethyl)-3-((4-methoxyphenyl)(methyl)amino)-5-methylpyrrolidine-1-carboxylate |