Online Database of Chemicals from Around the World
| Ningbo Dongbo New Energy Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (0574) 6329-6893 | |||
 |
alice@dobonewenergy.com | |||
 |
QQ chat | |||
 |
WeChat: 18668281331 | |||
 |
WhatsApp: 15824288686 | |||
| Chemical distributor since 2020 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Catalysts and additives >> Polymer |
|---|---|
| Name | 4,4'-(1-methylethylidene)bis-phenol polymer with 1,1'-sulfonylbis(4-chlorobenzene) |
| Molecular Structure | 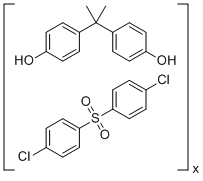 |
| Molecular Formula | (C15H16O2)x.(C12H8Cl2O2S)x |
| CAS Registry Number | 25154-01-2 |
| EC Number | 807-047-3 |
| SMILES | CC(C)(c1ccc(O)cc1)c1ccc(O)cc1.O=S(=O)(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |
|
4,4'-(1-methylethylidene)bis-phenol polymer with 1,1'-sulfonylbis(4-chlorobenzene) is a condensation polymer formed by the reaction between bisphenol A and 1,1'-sulfonylbis(4-chlorobenzene), a sulfone-containing aromatic compound. This polymer belongs to the family of polysulfones, which are high-performance thermoplastics known for their exceptional thermal stability, mechanical strength, and chemical resistance. The polymer backbone contains repeating units linked by sulfone (–SO2–) bridges and aromatic rings, resulting in a rigid and robust macromolecular structure. The synthesis of this polymer typically involves a nucleophilic aromatic substitution reaction where the phenolic hydroxyl groups of bisphenol A react with the chlorinated sulfone compound under basic conditions. This process results in the formation of ether linkages (–O–) connecting aromatic units through sulfone linkages. The polymerization is usually carried out at elevated temperatures in polar aprotic solvents to facilitate reaction kinetics and solubility of intermediates. Controlling reaction parameters such as temperature, monomer ratio, and reaction time allows for tuning molecular weight and polymer properties. Polysulfones derived from 4,4'-(1-methylethylidene)bis-phenol and 1,1'-sulfonylbis(4-chlorobenzene) exhibit high glass transition temperatures, typically above 180°C, which enables their use in applications requiring dimensional stability under heat. Their outstanding chemical resistance includes stability against hydrolysis, oxidation, and many organic solvents, making them suitable for harsh environments. These polymers find extensive application in membrane technology, particularly for gas separation, ultrafiltration, and reverse osmosis membranes. Their mechanical strength combined with chemical and thermal stability ensures durability and performance in filtration systems used in water treatment, gas purification, and medical devices. The polymer’s amorphous nature and tunable porosity contribute to its selective permeability and fouling resistance. In addition to membranes, polysulfones serve as engineering plastics in automotive, aerospace, and electrical industries. Components manufactured from this polymer include connectors, housings, and precision parts that require dimensional accuracy, flame retardancy, and resistance to sterilization processes. The inherent flame resistance of polysulfones, attributed to their aromatic and sulfone structures, complies with stringent safety standards. The polymer also plays a role in medical applications, where its biocompatibility and sterilization resistance enable use in devices such as dialysis membranes, surgical instruments, and dental appliances. Its transparency and mechanical robustness further broaden its utility in biomedical engineering. Environmental considerations for polysulfones focus on their thermoplastic recyclability, although their high thermal stability requires specialized recycling processes. The polymers are not biodegradable, but their resistance to degradation contributes to long service life in demanding applications. Research efforts explore the development of polysulfones with enhanced processability and incorporation of sustainable monomers to improve environmental footprint. Overall, the polymer formed from 4,4'-(1-methylethylidene)bis-phenol and 1,1'-sulfonylbis(4-chlorobenzene) represents a class of advanced materials with a combination of thermal, mechanical, and chemical properties suitable for high-performance engineering and biomedical applications. Its versatility and durability continue to drive innovation in polymer science and technology. |
| Market Analysis Reports |
| List of Reports Available for 4,4'-(1-methylethylidene)bis-phenol polymer with 1,1'-sulfonylbis(4-chlorobenzene) |