Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Organic raw materials >> Heterocyclic compound |
|---|---|
| Name | 2,2,6,6-Tetramethylpiperidinooxy |
| Synonyms | 2,2,6,6-Tetramethylpiperidine 1-oxyl; TEMPO |
| Molecular Structure | 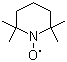 |
| Molecular Formula | C9H18NO |
| Molecular Weight | 156.25 |
| CAS Registry Number | 2564-83-2 |
| EC Number | 219-888-8 |
| SMILES | CC1(CCCC(N1[O])(C)C)C |
| Melting point | 36-40 ºC |
|---|---|
| Boiling point | 193 ºC |
| Flash point | 67 ºC |
| Hazard Symbols |

 GHS05;GHS07 Danger Details
GHS05;GHS07 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H314-H318-H335-H412 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P260-P261-P264-P264+P265-P271-P273-P280-P301+P330+P331-P302+P361+P354-P304+P340-P305+P354+P338-P316-P317-P319-P321-P363-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 3263 | ||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||
|
2,2,6,6-Tetramethylpiperidinooxy, abbreviated as TEMPO, was discovered in the late 1960s by German chemists. Initially, it was synthesized as a stable free radical for use in studying reaction mechanisms. However, its unique properties soon garnered attention beyond academia. TEMPO acts as a highly efficient catalyst in oxidation reactions, particularly in the conversion of alcohols to carbonyl compounds. Its ability to selectively oxidize primary alcohols to aldehydes and secondary alcohols to ketones makes it invaluable in organic synthesis. TEMPO is extensively used in polymer chemistry for the synthesis of functional polymers with controlled structures and properties. By mediating radical polymerization reactions, it enables the production of polymers with tailored molecular weights and architectures, essential for applications in materials science and drug delivery. In the paper industry, TEMPO is employed as a bleaching agent to improve the brightness and strength of pulp fibers. Its mild oxidation properties allow for selective removal of lignin and other impurities from wood pulp without damaging cellulose fibers, leading to the production of high-quality paper products. TEMPO exhibits antioxidant properties, making it useful in biomedical applications for scavenging free radicals and protecting cells from oxidative stress. Research suggests its potential in mitigating oxidative damage associated with neurodegenerative diseases and age-related disorders. TEMPO-modified electrodes are utilized in electrochemical sensors for detecting various analytes, including biomolecules, pollutants, and pharmaceuticals. Its stable redox behavior and high sensitivity enable precise and selective detection. TEMPO-based compounds are employed as food additives for preserving freshness and extending shelf life. By inhibiting lipid oxidation, they prevent rancidity and maintain the quality of packaged foods, such as oils, fats, and processed meats. References 2022. Electrocatalytic oxidative upgrading of biomass platform chemicals: from the aspect of reaction mechanism. Chemical communications (Cambridge, England), 58(8). DOI: 10.1039/d1cc06254a 2020. TEMPO-oxidized bacterial cellulose nanofiber membranes as high-performance separators for lithium-ion batteries. Carbohydrate Polymers, 229. DOI: 10.1016/j.carbpol.2019.115570 2009. Mechanistic insight into TEMPO-inhibited polymerisation: simultaneous determination of oxygen and inhibitor concentrations by EPR. Organic & Biomolecular Chemistry, 7(13). DOI: 10.1039/b905893a |
| Market Analysis Reports |
| List of Reports Available for 2,2,6,6-Tetramethylpiperidinooxy |